Exam 24: Nuclear Reactions and Their Applications
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
In this equation 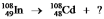 , what particle or type of radiation needs to be included on the right-hand side in order to balance it?
, what particle or type of radiation needs to be included on the right-hand side in order to balance it?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
D
Carbon-14 will emit a particle with an energy of 0.1565 MeV. What is this energy in joules?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
D
It is believed that two carbon-12 nuclei can react in the core of a supergiant star to form sodium-23 and hydrogen-1. Calculate the energy released from this reaction for each mole of hydrogen formed. The masses of carbon-12, sodium-23, and hydrogen-1 are 12.0000 amu, 22.989767 amu, and 1.007825, respectively. 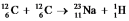
Free
(Multiple Choice)
4.9/5  (45)
(45)
Correct Answer:
C
The isotope 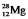 has a half-life of 21 hours. If a sample initially contains exactly 10,000 atoms of
has a half-life of 21 hours. If a sample initially contains exactly 10,000 atoms of 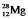 , approximately how many of these atoms will remain after one week?
, approximately how many of these atoms will remain after one week?
(Multiple Choice)
4.8/5  (37)
(37)
An isotope with a low value of N/Z will generally decay through
(Multiple Choice)
4.7/5  (40)
(40)
The nuclide Pb-210 undergoes three successive decays (beta, alpha, and beta, respectively) to form a stable nuclide. What are the three nuclides which form from Pb-210 in this decay series?
(Multiple Choice)
4.8/5  (32)
(32)
11 Select the nuclide that completes the following nuclear reaction: 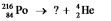
(Multiple Choice)
4.7/5  (29)
(29)
Exposure to 10 nCi for 10 minutes is more hazardous for a child than for an adult because
(Multiple Choice)
4.8/5  (39)
(39)
A 55-kg person exposed to thorium-234 receives 7.5 * 104 particles, each with an energy of 1.6 *10¯14 J. How many rads does the person receive?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following isotopes is most likely to be unstable?
(Multiple Choice)
4.9/5  (46)
(46)
Bombardment of uranium-238 nuclei by carbon-12 nuclei produces californium-246 and neutrons. Write a complete, balanced equation for this nuclear process.
(Short Answer)
4.7/5  (29)
(29)
Identify the missing species in the following nuclear transmutation: 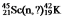
(Multiple Choice)
5.0/5  (42)
(42)
The isotope 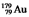 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 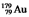 , approximately how many such atoms were present 30 seconds earlier?
, approximately how many such atoms were present 30 seconds earlier?
(Multiple Choice)
4.8/5  (41)
(41)
Detection of radiation by a Geiger-Müller counter depends on
(Multiple Choice)
4.8/5  (29)
(29)
The isotopes of promethium, 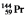 and
and 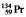 , are unstable, and lie on opposite sides of the "line of stability." Which of the following combinations is most likely to represent the type of decay for these isotopes?
, are unstable, and lie on opposite sides of the "line of stability." Which of the following combinations is most likely to represent the type of decay for these isotopes?
(Multiple Choice)
4.8/5  (36)
(36)
Explain how the number of protons and neutrons in a radioactive nucleus can be used to predict its probable mode of decay. Illustrate your answer with a schematic graph, properly labeled, showing stable nuclides (nuclei) in relation to number of protons and neutrons.
(Essay)
4.8/5  (42)
(42)
Which of the following types of radioactive decay does not produce new element?
(Multiple Choice)
4.8/5  (49)
(49)
Identify the missing species in the following nuclear transmutation: 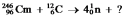
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)