Exam 2: The Components of Matter
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
What is the name of the acid formed when H2S gas is dissolved in water?
(Multiple Choice)
4.9/5  (40)
(40)
Calcium hydroxide is used in mortar, plaster and cement. What is its formula?
(Multiple Choice)
4.8/5  (36)
(36)
State the two important experimental results (and the names of the responsible scientists) which enabled the mass of the electron to be determined.
(Essay)
4.9/5  (44)
(44)
Which one of the following groups does not contain any metals?
(Multiple Choice)
4.8/5  (32)
(32)
Atoms X, Y, Z, and R have the following nuclear compositions:
 Which two are isotopes?
Which two are isotopes?
(Multiple Choice)
4.8/5  (42)
(42)
Determine the molecular mass of iron (III) bromide hexahydrate, a substance used as a catalyst in organic reactions.
(Multiple Choice)
4.7/5  (48)
(48)
Who is credited with first measuring the charge of the electron?
(Multiple Choice)
4.8/5  (38)
(38)
Tetrasulfur dinitride decomposes explosively when heated. What is its formula?
(Multiple Choice)
4.9/5  (33)
(33)
Diiodine pentaoxide is used as an oxidizing agent that converts carbon monoxide to carbon dioxide. What is its chemical formula?
(Multiple Choice)
4.9/5  (46)
(46)
Which, if any, of the following elements do not occur in the major classes of organic compounds?
(Multiple Choice)
4.8/5  (38)
(38)
Bromine is the only nonmetal that is a liquid at room temperature. Consider the isotope bromine-81, 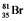 . Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
. Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
(Multiple Choice)
4.8/5  (38)
(38)
When an atom is represented by the symbol 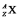 , the value of A is the
, the value of A is the
(Multiple Choice)
5.0/5  (35)
(35)
Ammonium sulfate, (NH4)2SO4, is a fertilizer widely used as a source of nitrogen. Calculate its molecular mass.
(Multiple Choice)
4.8/5  (28)
(28)
Which one of the following combinations of names and formulas of ions is incorrect?
(Multiple Choice)
4.8/5  (26)
(26)
Showing 21 - 40 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)