Exam 14: Oxidation-Reduction Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
A voltaic cell is prepared in which copper metal is oxidized to Cu(II), and silver ion is reduced to silver metal.Which of the following represents the equation for the reaction that occurs at the anode?
(Multiple Choice)
4.9/5  (42)
(42)
Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Which of the following statements is correct?
(Multiple Choice)
4.9/5  (33)
(33)
All of these statements concerning oxidation are correct except that
(Multiple Choice)
4.9/5  (36)
(36)
What is the oxidation number of boron in sodium tetraborate, Na2B4O7?
(Multiple Choice)
4.8/5  (43)
(43)
The reaction that occurs in an alkaline battery is as follows: Zn(s) + MnO2(s) + H2O(l) ZnO(s) + Mn(OH)2(s) The substance that is oxidized in this battery is:
(Multiple Choice)
4.8/5  (41)
(41)
Consider the following reaction: Mg(s) + ZnSO4(aq) MgSO4(aq) + Zn(s) Which of the following statements regarding this reaction is correct?
(Multiple Choice)
4.9/5  (43)
(43)
The figure shows the electrolysis of molten NaI.What reaction occurs at the anode of this cell? 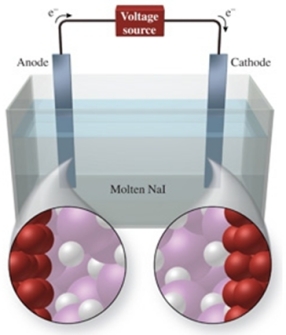
(Multiple Choice)
4.7/5  (46)
(46)
For the following reaction, what is the oxidizing agent? 2Cl2(g) + C(s) + 2H2O(l) CO2(g) + 4HCl(aq)
(Multiple Choice)
4.8/5  (48)
(48)
A lead-acid battery that is used in cars and trucks is powered by the reaction: PbO2(s) + Pb(s) + 2H2SO4(aq) 2PbSO4(s) + H2O(l) The substance that is the reducing agent in this battery is:
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements regarding oxidation-reduction reactions is correct?
(Multiple Choice)
4.7/5  (42)
(42)
A species that causes a decrease in the oxidation number of another substance is called a reducing agent.
(True/False)
4.8/5  (40)
(40)
Consider the reaction: H3AsO3(aq) + BiO3-(aq) H3AsO4(aq) + Bi(s) When this equation is balanced in acidic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
(Multiple Choice)
4.8/5  (45)
(45)
Consider the reaction: Sn2+(aq) + 2Fe3+(aq) Sn4+(aq) + 2Fe2+(aq) Which species is oxidized, and how many electrons are transferred per SN2+ ion that reacts?
(Multiple Choice)
4.8/5  (38)
(38)
In which compound does phosphorus have an oxidation number of 3+?
(Multiple Choice)
4.8/5  (19)
(19)
Consider the half-reaction Cr3+(aq) Cr2O72-(aq).When the equation is balanced in acid solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
(Multiple Choice)
4.9/5  (41)
(41)
Given the following reaction in a voltaic cell: Zn(s) + Ni2+(aq) Ni(s) + Zn2+(aq) In this cell, Zn(s) is called the
(Multiple Choice)
4.7/5  (35)
(35)
Consider the reaction: Al(s) + H2O(l) Al(OH)4-(aq) + H2(g) When this equation is balanced in basic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
(Multiple Choice)
4.9/5  (29)
(29)
Consider the reaction: N2(g) + 3H2(g) 2NH3(g) Which of the following statements is correct?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 117
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)