Exam 14: Oxidation-Reduction Reactions
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Equations that are written to represent either an oxidation reaction or a reduction reaction are called half-reactions.
(True/False)
4.7/5  (44)
(44)
The figure shows the electrolysis of molten NaI.What reaction occurs at the anode of this cell? 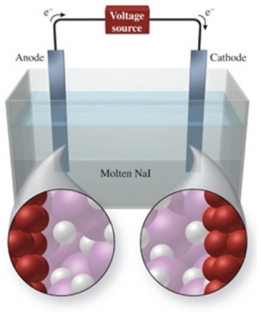
(Multiple Choice)
4.9/5  (37)
(37)
The figure shows a molecular-level representation of the following voltaic cell: Mg(s) + Sn2+(aq) Mg2+(aq) + Sn(s) When drawing the "after" representation one would note that: 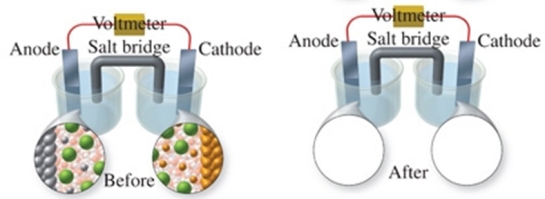
(Multiple Choice)
4.8/5  (36)
(36)
Consider the half-reaction NO3-(aq) NO(aq).When the equation is balanced in acid solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
(Multiple Choice)
4.9/5  (36)
(36)
What are the oxidation numbers of the atoms in the AsO43- ion?
(Multiple Choice)
4.9/5  (35)
(35)
The following reaction occurs in a lead storage battery: PbO2(s) + Pb(s) + H2SO4(aq) ⇌ 2PbSO4(s) + 2H2O(l) What happens to the sulfuric acid in a lead storage battery when the battery is being discharged?
(Multiple Choice)
4.7/5  (39)
(39)
The products of the electrolysis of molten magnesium chloride are
(Multiple Choice)
4.7/5  (40)
(40)
A voltaic cell is prepared in which aluminum metal is oxidized to Al3+, and nickel(II) is reduced to nickel metal.Which of the following represents the correctly balanced equation for this reaction?
(Multiple Choice)
4.9/5  (33)
(33)
What are the oxidation numbers of the atoms in the Cr2O72- ion?
(Multiple Choice)
4.9/5  (31)
(31)
Given the following information about the activity series, select element(s) that could be used to protect the iron in a steel evaporative (swamp) cooler from corrosion.Cu < H2 < Sn < Fe < Zn < Mg
(Multiple Choice)
4.8/5  (36)
(36)
What are the oxidation numbers of the atoms in the MnO4- ion?
(Multiple Choice)
4.8/5  (37)
(37)
Given the following information about the activity series, select element(s) that could be used to protect the iron in a steel ship's hull from corrosion.Cu < H2 < Sn < Fe < Zn < Mg
(Multiple Choice)
4.9/5  (40)
(40)
A voltaic cell is prepared in which copper metal is oxidized to Cu(II), and silver ion is reduced to silver metal.Which of the following represents the correctly balanced equation for this reaction?
(Multiple Choice)
4.9/5  (42)
(42)
In which of the following choices is the oxidation number incorrect?
(Multiple Choice)
4.8/5  (36)
(36)
Batteries are electrochemical cells that are constructed so that a barrier separates the oxidation reaction from the reduction reaction.
(True/False)
4.8/5  (39)
(39)
A steel utility pole (made primarily of iron) can be protected from oxidation by attaching a piece of copper, or some other relatively inactive metal, to it.
(True/False)
4.9/5  (31)
(31)
Showing 101 - 117 of 117
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)