Exam 13: Acids and Bases
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Select the pair that consists of a base and its conjugate acid in that order.
(Multiple Choice)
4.8/5  (39)
(39)
Rank the following 0.100 M solutions in order of increasing H3O+ concentration: HCN, Ka = 6.2 x 10-10; NH4+, Ka = 5.6 x 10-10; CH3CO2H, Ka = 1.8 x 10-5
(Multiple Choice)
4.8/5  (44)
(44)
The image shows a molecular-level representation of part of a solution after HF is dissolved in water.(Besides water, there are 6 HF molecules, 1 H3O+ ion, and 1 F- ion present in the solution.) Which of the following best describes HF? 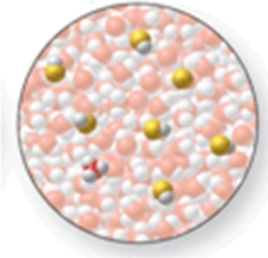
(Multiple Choice)
4.9/5  (37)
(37)
When acetate ion, CH3CO2-, reacts with water, what are the products?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements regarding acids is incorrect?
(Multiple Choice)
4.8/5  (41)
(41)
When carbonate ion, CO32-, reacts with water, what are the products?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the pOH of a solution that has [H3O+] = 5.2 x 10-7 M.
(Multiple Choice)
4.9/5  (35)
(35)
The image shows a molecular-level representation of part of a solution after HCl is dissolved in water.What best describes this image? (Besides water, there are 6 H3O+ ions and 6 Cl- ions present in the solution.) Which of the following best describes HCl? 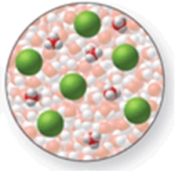
(Multiple Choice)
4.9/5  (39)
(39)
The Ka for acetic acid is 1.8 x 10-5.Which of the following statements best describes the pH of a 0.010 M solution of acetic acid?
(Multiple Choice)
4.9/5  (44)
(44)
When the following reaction goes in the reverse direction (from products to reactants), what is the acid? HF(aq) + H2O(l) ⇌ F-(aq) + H3O+(aq)
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the pH of a solution that has [H3O+] = 1.0 x 10-6 M.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following equations represents the behavior of HCO3- as an acid?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 61 - 80 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)