Exam 10: The Shapes of Molecules
Exam 1: Keys to the Study of Chemistry: Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter101 Questions
Exam 3: Stoichiometry of Formulas and Equations72 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory98 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes95 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements103 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions98 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and Reaction Direction85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work101 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds81 Questions
Exam 24: Nuclear Reactions and Their Applications82 Questions
Select questions type
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90° bond angle in it. (In each case except water, the central atom is the first one in the formula.)
(Multiple Choice)
4.7/5  (35)
(35)
What is the molecular shape of BCl3 as predicted by the VSEPR theory?
(Multiple Choice)
4.8/5  (46)
(46)
Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.)
(Multiple Choice)
4.9/5  (34)
(34)
According to VSEPR theory, a molecule with the general formula AX4E2 will have a __________ molecular shape.
(Multiple Choice)
4.9/5  (30)
(30)
How many electron pairs are shared between the carbon atoms in C2H4?
(Multiple Choice)
4.9/5  (38)
(38)
Predict the actual bond angles in SF3+ using the VSEPR theory.
(Multiple Choice)
4.7/5  (36)
(36)
In which of the following does the nitrogen atom have a formal charge of −1?
(Multiple Choice)
4.8/5  (39)
(39)
What is the molecular shape of NO2− as predicted by the VSEPR theory?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following Lewis structures is definitely incorrect? 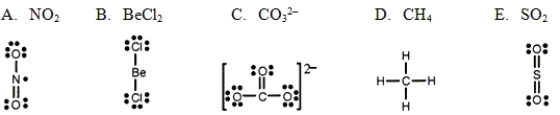
(Multiple Choice)
4.7/5  (38)
(38)
What is the molecular shape of BeH2 as predicted by the VSEPR theory?
(Multiple Choice)
4.8/5  (34)
(34)
According to VSEPR theory, a molecule with the general formula AX2E will have a __________ molecular shape.
(Multiple Choice)
4.9/5  (34)
(34)
What is the molecular shape of ClCN as predicted by the VSEPR theory? (Carbon is the central atom.)
(Multiple Choice)
4.8/5  (38)
(38)
What is the molecular shape of the thiocyanate anion, SCN−, as predicted by the VSEPR theory? (Carbon is the central atom.)
(Multiple Choice)
4.8/5  (40)
(40)
According to VSEPR theory, a molecule with the general formula AX6 will have a __________ molecular shape.
(Multiple Choice)
4.9/5  (33)
(33)
Predict the ideal bond angles around nitrogen in N2F2 using the molecular shape given by the VSEPR theory. (The two N atoms are the central atoms.)
(Multiple Choice)
4.8/5  (32)
(32)
What is the molecular shape of SCl3F as predicted by the VSEPR theory?
(Multiple Choice)
4.7/5  (36)
(36)
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
(Multiple Choice)
4.7/5  (32)
(32)
In a Lewis structure for a molecule or ion, the sum of the formal charges on the atoms is equal to the charge on the molecule or ion.
(True/False)
4.7/5  (27)
(27)
Which one of the following molecules and ions will have a planar geometry?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 21 - 40 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)