Exam 10: The Atomic Nucleus and Radioactivity
Exam 1: About Science24 Questions
Exam 2: Describing Motion74 Questions
Exam 3: Newtons Laws of Motion124 Questions
Exam 4: Momentum and Energy133 Questions
Exam 5: Gravity88 Questions
Exam 6: Heat98 Questions
Exam 7: Electricity and Magnetism107 Questions
Exam 8: Waves-Sound and Light124 Questions
Exam 9: Atoms and the Periodic Table133 Questions
Exam 10: The Atomic Nucleus and Radioactivity129 Questions
Exam 11: Investigating Matter87 Questions
Exam 12: Chemical Bonds and Mixtures248 Questions
Exam 13: Chemical Reactions252 Questions
Exam 14: Organic Compounds107 Questions
Exam 15: The Basic Unit of Life-The Cell37 Questions
Exam 16: Genetics40 Questions
Exam 17: The Evolution of Life35 Questions
Exam 18: Diversity of Life on Earth41 Questions
Exam 19: Human Biology Icontrol and Development39 Questions
Exam 20: Human Biology Iicare and Maintenance37 Questions
Exam 21: Ecology36 Questions
Exam 22: Plate Tectonics83 Questions
Exam 23: Rocks and Minerals59 Questions
Exam 24: Earths Surfaceland and Water72 Questions
Exam 25: Surface Processes62 Questions
Exam 26: Weather72 Questions
Exam 27: Environmental Geology52 Questions
Exam 28: The Solar System98 Questions
Exam 29: The Universe111 Questions
Select questions type
Complete the following nuclear equation: 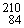 Po → ?? +
Po → ?? + 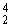 He
He
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
A certain radioactive element has a half-life of one hour. If you start with a 1-g sample of the element at noon, how much of this same element will be left at 3:00 PM?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
C
Why is the combination of two protons and two neutrons stable, but two protons and one neutron is not?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
Which of the following statements best describes the strong nuclear force?
(Multiple Choice)
4.8/5  (45)
(45)
Light nuclei can be split. For example, a deuteron, which is a proton-neutron combination, can split into a separate proton and separate neutron. Does such a process yield energy or cost energy? Why?
(Multiple Choice)
4.8/5  (35)
(35)
The element uranium (U, atomic no. = 92)often has 146 (or more)neutrons but it will still undergo radioactive decay. Which statement might best describe why?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following does not describe a nuclear fission reaction?
(Multiple Choice)
4.9/5  (39)
(39)
The isotope Cesium-137, which has a half-life of 30 years, is a product of nuclear power plants. How long will it take for this isotope to decay to about one-sixteenth its original amount?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following types of radiation might be best for medical imaging?
(Multiple Choice)
4.8/5  (39)
(39)
What are the three types of rays produced in radioactive decay? Which will penetrate farther into material?
(Essay)
4.8/5  (39)
(39)
Why might the following nuclear reaction not be very good for energy production in a fission reactor? 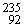 U →
U → 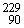 Th +
Th + 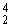 He + 3 neutrons
He + 3 neutrons
(Multiple Choice)
4.7/5  (29)
(29)
Radioactivity is a tendency for an element or a material to
(Multiple Choice)
4.9/5  (25)
(25)
Why is carbon better than lead as a moderator in nuclear reactors?
(Multiple Choice)
4.8/5  (28)
(28)
If three samples have the half-lives listed below, which is the most radioactive?
(Multiple Choice)
4.8/5  (37)
(37)
People who work around radioactivity wear film badges to monitor the amount of radiation that reaches their bodies. These badges consist of small pieces of photographic film enclosed in a light-proof wrapper. What kind of radiation do these devices monitor?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following is a major advantage of nuclear-fission-based power plants?
(Multiple Choice)
4.8/5  (36)
(36)
If a nucleus of 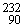 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following professions would likely have the highest occupational exposure to radiation per year?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 1 - 20 of 129
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)