Exam 10: The Atomic Nucleus and Radioactivity
Exam 1: About Science24 Questions
Exam 2: Describing Motion74 Questions
Exam 3: Newtons Laws of Motion124 Questions
Exam 4: Momentum and Energy133 Questions
Exam 5: Gravity88 Questions
Exam 6: Heat98 Questions
Exam 7: Electricity and Magnetism107 Questions
Exam 8: Waves-Sound and Light124 Questions
Exam 9: Atoms and the Periodic Table133 Questions
Exam 10: The Atomic Nucleus and Radioactivity129 Questions
Exam 11: Investigating Matter87 Questions
Exam 12: Chemical Bonds and Mixtures248 Questions
Exam 13: Chemical Reactions252 Questions
Exam 14: Organic Compounds107 Questions
Exam 15: The Basic Unit of Life-The Cell37 Questions
Exam 16: Genetics40 Questions
Exam 17: The Evolution of Life35 Questions
Exam 18: Diversity of Life on Earth41 Questions
Exam 19: Human Biology Icontrol and Development39 Questions
Exam 20: Human Biology Iicare and Maintenance37 Questions
Exam 21: Ecology36 Questions
Exam 22: Plate Tectonics83 Questions
Exam 23: Rocks and Minerals59 Questions
Exam 24: Earths Surfaceland and Water72 Questions
Exam 25: Surface Processes62 Questions
Exam 26: Weather72 Questions
Exam 27: Environmental Geology52 Questions
Exam 28: The Solar System98 Questions
Exam 29: The Universe111 Questions
Select questions type
Which of the following statements about fusion is the most accurate?
(Multiple Choice)
4.9/5  (37)
(37)
Just after an alpha particle leaves the nucleus, would you expect it to speed up? Why?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements best describes the role of neutrons in the nucleus?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following nuclear equations correctly describes alpha emission?
(Multiple Choice)
4.7/5  (36)
(36)
When  Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
(Multiple Choice)
4.9/5  (33)
(33)
A pair of protons in an atomic nucleus repel each other, but they are also attracted to each other. Why?
(Multiple Choice)
4.9/5  (40)
(40)
Uranium-235 releases an average of 2.5 neutrons per fission, while plutonium-239 releases an average of 2.7 neutrons per fission. Which of these elements might you therefore expect to have the smaller critical mass?
(Multiple Choice)
4.8/5  (42)
(42)
Why does plutonium not occur in appreciable amounts in natural ore deposits?
(Multiple Choice)
4.9/5  (42)
(42)
Why might the following nuclear reaction not be very good for energy production in a fusion reactor? 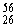 Fe +
Fe + 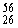 Fe →
Fe → 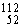 Te
Te
(Multiple Choice)
4.9/5  (40)
(40)
How many alpha particles are emitted in the series of radioactive decay events from a U-238 nucleus to a Pb-206 nucleus?
(Multiple Choice)
4.9/5  (40)
(40)
In bombarding atomic nuclei with proton "bullets," the protons must be accelerated to high energies to make contact with the target nuclei
(Multiple Choice)
4.8/5  (33)
(33)
The original reactor built in 1942 was just "barely" critical because the natural uranium that was used contained less than 1% of the fissionable isotope U-235 (half-life 713 million years). What if, in 1942, the Earth had been 9 billion years old instead of 4.5 billion years old? Would this reactor have reached critical stage with natural uranium? Why?
(Multiple Choice)
4.7/5  (39)
(39)
When 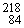 Po emits a beta particle, it transforms into a new element. What are the atomic number and atomic mass of this new element?
Po emits a beta particle, it transforms into a new element. What are the atomic number and atomic mass of this new element?
(Multiple Choice)
4.8/5  (32)
(32)
If a nucleus of 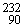 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 129
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)