Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions
Exam 1: Structure and Bonding:acids and Bases41 Questions
Exam 2: Alkanes: the Nature of Organic Compounds44 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions40 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds52 Questions
Exam 6: Sterechemistry at Tetrahedral Centers39 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations40 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs36 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions63 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions38 Questions
Exam 12: Amines32 Questions
Exam 13: Structure Determination65 Questions
Exam 14: Biomolecules: Carbohydrates48 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins50 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids50 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways40 Questions
Select questions type
Instructions: Provide IUPAC names for each structure below.
-Name: 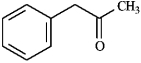
Free
(Short Answer)
4.8/5  (49)
(49)
Correct Answer:
phenylacetone or 1-phenylpropan-2-one
The nucleophillic addition of water to an aldehyde or ketone
Free
(Multiple Choice)
4.7/5  (43)
(43)
Correct Answer:
B
Instructions: Consider the reaction below to answer the following question. 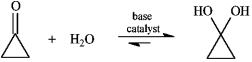 -Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
-Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
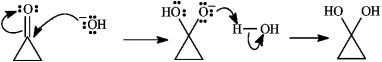
Instructions: Provide IUPAC names for each structure below.
-Name: 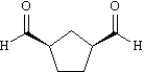
(Short Answer)
4.8/5  (39)
(39)
Propose a synthesis of Dimestrol starting from p-methoxypropiophenone as the only source of carbon. 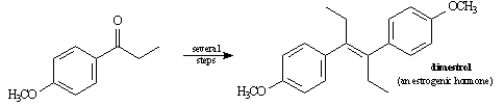
(Essay)
4.9/5  (35)
(35)
Instructions: Predict the products from the information given for the following question(s).
-Predict: 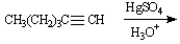
(Essay)
4.9/5  (42)
(42)
Instructions: Draw structures corresponding to each of the following names.
-Draw:
2,2-dimethylcyclopentane-1,3-dione
(Essay)
4.9/5  (35)
(35)
Instructions: Predict the products from the information given for the following question(s).
-Predict: 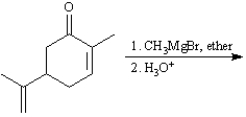
(Essay)
4.8/5  (43)
(43)
Instructions: Consider the reaction below to answer the following question. 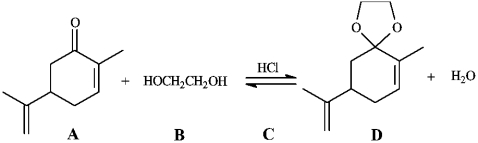 -Refer to instructions. The product of this reaction is called:
-Refer to instructions. The product of this reaction is called:
(Multiple Choice)
4.8/5  (35)
(35)
Instructions: Draw structures corresponding to each of the following names.
-Draw:
trans-3-isopropylcyclohexanecarbaldehyde
(Essay)
4.9/5  (31)
(31)
Instructions: Predict the products from the information given for the following question(s).
-Predict: 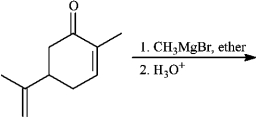
(Essay)
4.9/5  (37)
(37)
Instructions: Draw structures corresponding to each of the following names.
-Draw:
cyclohex-2-enone
(Essay)
4.8/5  (38)
(38)
Instructions: , -Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the carbon, as shown below. Use this information to answer the following question(s). 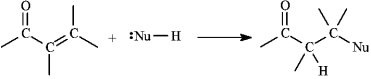 -Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
-Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
(Essay)
4.9/5  (34)
(34)
Instructions: Draw structures corresponding to each of the following names.
-Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)
(Essay)
4.9/5  (40)
(40)
Instructions: Consider the reaction below to answer the following question.  -Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
-Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
(Short Answer)
4.9/5  (45)
(45)
Instructions: Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of -hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated. 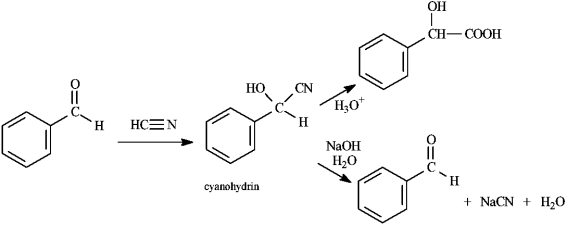 -Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
-Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 32
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)