Exam 1: Structure and Bonding:acids and Bases
Exam 1: Structure and Bonding:acids and Bases41 Questions
Exam 2: Alkanes: the Nature of Organic Compounds44 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions40 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds52 Questions
Exam 6: Sterechemistry at Tetrahedral Centers39 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations40 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs36 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions63 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions38 Questions
Exam 12: Amines32 Questions
Exam 13: Structure Determination65 Questions
Exam 14: Biomolecules: Carbohydrates48 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins50 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids50 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways40 Questions
Select questions type
Instructions: Refer to the following equation to answer the question(s) below.  -Refer to instructions. Will this reaction take place as written in the forward direction? Explain.
-Refer to instructions. Will this reaction take place as written in the forward direction? Explain.
Free
(Essay)
4.7/5  (42)
(42)
Correct Answer:
No, the reaction will not take place as written because the strongest acid reacts with the strongest base to give the weakest conjugate acid and the weakest conjugate base. D (pKa = 15.7) is a stronger acid than A (pKa = 18).
Write an equation for the reaction of boron trifluoride, an important reagent in organic chemistry, with trimethylamine. Represent the movement of electrons with a curved arrow, and show the formal charges on the atoms in the product. 
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
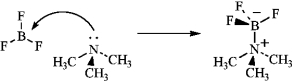
Which of the following best represents the shape of a sp3 hybrid orbital of carbon? 
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
C
How many total valence electrons are represented in the following electron configuration? 1s22s22px2 2py2 2pz1 or 1s22s22p5
(Multiple Choice)
4.9/5  (35)
(35)
How many nonbonding electron pairs are in the structure shown below? 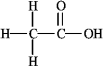
(Multiple Choice)
4.8/5  (32)
(32)
Instructions: Propose a structure for a molecule that meets the following description.
-Refer to instructions. Contains only two sp3 hybridized carbons and two sp hybridized carbons.
(Essay)
4.9/5  (39)
(39)
Instructions: Consider the reaction below to answer the following question.  -Refer to instructions. Using the curved arrow formalism, show the flow of electrons for this reaction.
-Refer to instructions. Using the curved arrow formalism, show the flow of electrons for this reaction.
(Essay)
5.0/5  (40)
(40)
Instructions: Consider the two structures below to answer the following question.
CH3CH2OH CH3OCH3
-In the two structures shown below, what do the positions labeled with the arrow have in common? 
(Multiple Choice)
4.9/5  (39)
(39)
Which is the strongest base (pKa values given for conjugate acid)?
(Multiple Choice)
4.9/5  (37)
(37)
In drawing the Lewis structure for an organic compound, the carbon atoms should always be shown with
(Multiple Choice)
4.9/5  (31)
(31)
Instructions: Use the convention / + and the crossed arrow  to show the direction of the expected polarity of the indicated bonds in the following compound(s).
-Refer to instructions. A C-O bond in tetrahydrofuran,
to show the direction of the expected polarity of the indicated bonds in the following compound(s).
-Refer to instructions. A C-O bond in tetrahydrofuran, 
(Essay)
4.7/5  (39)
(39)
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules. Which one of the following Kekulé structures is not consistent with valence rules?
(Multiple Choice)
4.9/5  (38)
(38)
Use the curved arrow method to show the electron movement, and label the acid, base, conjugate acid, and conjugate base. 
(Essay)
4.9/5  (38)
(38)
Consider the structure of acetic acid shown below. 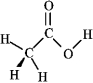 In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
(Multiple Choice)
4.9/5  (41)
(41)
Instructions: Consider the two structures below to answer the following question.
CH3CH2OH CH3OCH3
-Refer to instructions. Which of the following correctly describes the structure of these compounds?
(Multiple Choice)
4.9/5  (19)
(19)
Instructions: Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. Use the structure of indole, below, to answer the following question(s).  -Refer to instructions. Indole can function as a Brønsted-Lowry acid in the presence of strong bases. Formulate a reaction, using a generic base (:B-), showing electron flow with arrows, that demonstrates this reactivity of indole.
-Refer to instructions. Indole can function as a Brønsted-Lowry acid in the presence of strong bases. Formulate a reaction, using a generic base (:B-), showing electron flow with arrows, that demonstrates this reactivity of indole.
(Essay)
4.8/5  (34)
(34)
The following shows an intermediate used in a Grignard synthesis. Which atom will inductively donate electrons in this species? 
(Multiple Choice)
4.8/5  (37)
(37)
Instructions: Label the acid and base in each reaction below.
-Label: 
(Short Answer)
4.8/5  (45)
(45)
Showing 1 - 20 of 41
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)