Exam 5: Light and Matter: Reading Messages from the Cosmos
Exam 1: A Modern View of the Universe105 Questions
Exam 2: Discovering the Universe for Yourself136 Questions
Exam 3: The Science of Astronomy107 Questions
Exam 4: Making Sense of the Universe: Understanding Motion, Energy, and Gravity125 Questions
Exam 5: Light and Matter: Reading Messages from the Cosmos140 Questions
Exam 6: Telescopes: Portals of Discovery80 Questions
Exam 7: Our Planetary System87 Questions
Exam 8: Formation of the Solar System89 Questions
Exam 9: Planetary Geology: Earth and the Other Terrestrial Worlds135 Questions
Exam 10: Planetary Atmospheres: Earth and the Other Terrestrial Worlds154 Questions
Exam 11: Jovian Planet Systems109 Questions
Exam 12: Asteroids, Comets, and Dwarf Planets: Their Nature, Orbits, and Impacts112 Questions
Exam 13: Other Planetary Systems: The New Science of Distant Worlds93 Questions
Exam 14: Our Star115 Questions
Exam 15: Surveying the Stars141 Questions
Exam 16: Star Birth103 Questions
Exam 17: Star Stuff122 Questions
Exam 18: The Bizarre Stellar Graveyard117 Questions
Exam 19: Our Galaxy106 Questions
Exam 20: Galaxies and the Foundations of Modern Cosmology96 Questions
Exam 21: Galaxy Evolution90 Questions
Exam 22: The Birth of the Universe91 Questions
Exam 23: Dark Matter, Dark Energy, and the Fate of the Universe105 Questions
Exam 24: Life in the Universe108 Questions
Exam 26: Space and Time82 Questions
Exam 27: Spacetime and Gravity69 Questions
Exam 28: Building Blocks of the Universe78 Questions
Select questions type
How does the spectrum of a molecule differ from the spectrum of an atom?
(Multiple Choice)
4.9/5  (40)
(40)
What do we mean when we say that electron energy levels in atoms are quantized?
(Essay)
4.8/5  (38)
(38)
Consider an atom of carbon in which the nucleus contains 6 protons and 7 neutrons.What is its atomic number and atomic mass number?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements about thermal radiation is always true?
(Multiple Choice)
4.8/5  (43)
(43)
Suppose that Star X and Star Y both have redshifts,but Star X has a larger redshift than Star Y.What can you conclude?
(Multiple Choice)
4.8/5  (43)
(43)
Suppose you know the frequency of a photon and the speed of light.What else can you determine about the photon?
(Multiple Choice)
4.8/5  (40)
(40)
We can learn a lot about the properties of a star by studying its spectrum.All of the following statements are true except one.Which one?
(Multiple Choice)
4.8/5  (38)
(38)
Suppose that two stars are identical in every way-for example,same distance,same mass,same temperature,same chemical composition,and same speed relative to Earth-except that one star rotates faster than the other.Spectroscopically,how could you tell the stars apart?
(Multiple Choice)
5.0/5  (37)
(37)
Grass is green because it absorbs green light,reflecting all other colors.
(True/False)
4.9/5  (41)
(41)
When an atom absorbs a photon containing energy,any of the following can happen except which?
(Multiple Choice)
4.9/5  (36)
(36)
Suppose you have a chunk of water ice.Describe what happens to it,in terms of phases,as you raise the temperature to millions of degrees.
(Essay)
5.0/5  (36)
(36)
The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
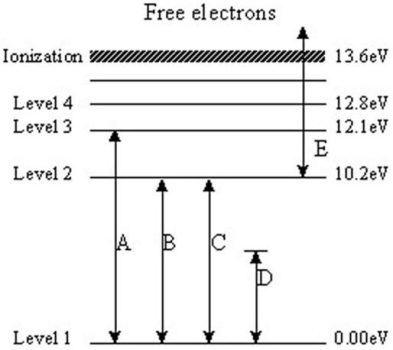 -Which transition,as shown,is not possible?
-Which transition,as shown,is not possible?
(Short Answer)
4.9/5  (41)
(41)
Showing 121 - 140 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)