Exam 5: Light and Matter: Reading Messages from the Cosmos
Exam 1: A Modern View of the Universe105 Questions
Exam 2: Discovering the Universe for Yourself136 Questions
Exam 3: The Science of Astronomy107 Questions
Exam 4: Making Sense of the Universe: Understanding Motion, Energy, and Gravity125 Questions
Exam 5: Light and Matter: Reading Messages from the Cosmos140 Questions
Exam 6: Telescopes: Portals of Discovery80 Questions
Exam 7: Our Planetary System87 Questions
Exam 8: Formation of the Solar System89 Questions
Exam 9: Planetary Geology: Earth and the Other Terrestrial Worlds135 Questions
Exam 10: Planetary Atmospheres: Earth and the Other Terrestrial Worlds154 Questions
Exam 11: Jovian Planet Systems109 Questions
Exam 12: Asteroids, Comets, and Dwarf Planets: Their Nature, Orbits, and Impacts112 Questions
Exam 13: Other Planetary Systems: The New Science of Distant Worlds93 Questions
Exam 14: Our Star115 Questions
Exam 15: Surveying the Stars141 Questions
Exam 16: Star Birth103 Questions
Exam 17: Star Stuff122 Questions
Exam 18: The Bizarre Stellar Graveyard117 Questions
Exam 19: Our Galaxy106 Questions
Exam 20: Galaxies and the Foundations of Modern Cosmology96 Questions
Exam 21: Galaxy Evolution90 Questions
Exam 22: The Birth of the Universe91 Questions
Exam 23: Dark Matter, Dark Energy, and the Fate of the Universe105 Questions
Exam 24: Life in the Universe108 Questions
Exam 26: Space and Time82 Questions
Exam 27: Spacetime and Gravity69 Questions
Exam 28: Building Blocks of the Universe78 Questions
Select questions type
Which of the following statements about X-rays and radio waves is not true?
(Multiple Choice)
4.8/5  (43)
(43)
Lines of a particular element appear at the same wavelength in both emission and absorption line spectra.
(True/False)
4.7/5  (40)
(40)
Which of the following statements about electrons is not true?
(Multiple Choice)
4.9/5  (29)
(29)
Consider an atom of oxygen in which the nucleus contains 8 protons and 8 neutrons.If it is doubly ionized,what is the charge of the oxygen ion and how many electrons remain in the ion?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following objects is not a close approximation of a thermal emitter?
(Multiple Choice)
4.9/5  (35)
(35)
Emission lines from different ionization states of the same element appear in the same place in the spectrum.
(True/False)
4.7/5  (36)
(36)
Process of Science: The theory of thermal radiation says that hot objects are bluer than cool objects.Does it depend on what the object is made of? How can you test this?
(Essay)
4.8/5  (35)
(35)
When light reflects off an object,what is the relation between the angle of incidence and the angle of reflection?
(Multiple Choice)
4.8/5  (40)
(40)
The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
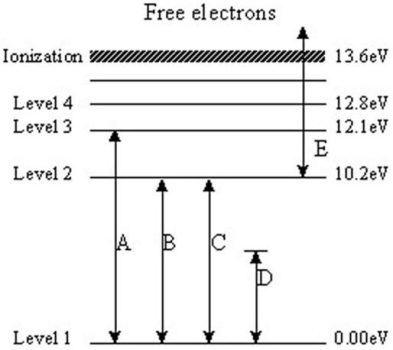 -Which transition represents an electron that absorbs a photon with 10.2 eV of energy?
-Which transition represents an electron that absorbs a photon with 10.2 eV of energy?
(Short Answer)
4.9/5  (39)
(39)
You observe the same spectral line in two stars that are identical in every way except that one rotates faster than the other.How does the spectral line differ between the two?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 21 - 40 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)