Exam 1: Matter, Measurements, and Calculations
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
If a 200 gram sample of water is partially frozen forming 40 g of ice, than 80% of the original sample is still a liquid.
Free
(True/False)
4.8/5  (37)
(37)
Correct Answer:
True
After heating, a pure substance, A, is found to produce both B and C. What can be said about the substance A?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
The prefix centi- denotes what fraction of a basic unit?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
Express the following "generic number" in scientific notation. 0.0000XXX
(Multiple Choice)
4.8/5  (38)
(38)
Ethanol (ethyl alcohol)has a density of 0.789 g\mL at 25°C. If 75.5 g of ethanol is needed for a reaction, what volume in mL should be added to the reaction container?
(Multiple Choice)
4.8/5  (36)
(36)
Based on data obtained in an experiment, to determine the density of a metal, the following calculation is carried out. Express the answer to the correct number of significant figures. 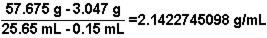
(Multiple Choice)
5.0/5  (36)
(36)
Which of the following terms correctly applies to a molecule of CO2?
(Multiple Choice)
5.0/5  (32)
(32)
Which of the following is an example of a homogeneous mixture?
(Multiple Choice)
4.8/5  (27)
(27)
If a student completes 5 problems out of a total of 8 on a pop quiz, what percentage of the quiz was completed?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following represents a physical change in matter?
(Multiple Choice)
4.9/5  (33)
(33)
One advantage of the Kelvin system is that it is impossible to have temperatures below zero.
(True/False)
5.0/5  (36)
(36)
The number twelve, representing a dozen, has two significant figures.
(True/False)
4.9/5  (29)
(29)
Do the following calculation and express the answer using correct scientific notation. ______ = (2.97 × 102)× (6.09 × 10 − 7)
(Multiple Choice)
4.7/5  (38)
(38)
Do the following calculation and express the answer using the correct number of significant figures. ______ = (1.21 × 10 − 3 + 1.3 × 10 − 3)× 6.453 × 102
(Multiple Choice)
4.9/5  (46)
(46)
A furnace delivers 8.0 × 104 BTU per hour. How many kilocalories per hour is this? (hint: 1 cal = 0.00397 BTU)
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)