Exam 6: The States of Matter
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Cohesive forces in matter are related to
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
What is the total pressure of a gaseous mixture that contains three gases with partial pressures of 0.845 atm, 120 torr and 210 mm Hg?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
B
The amount of pressure on a container at 100 ° C is twice the pressure on the container at 50 ° C.
Free
(True/False)
4.9/5  (45)
(45)
Correct Answer:
False
How does the state of a substance depend on the relative strengths of the cohesive forces and disruptive forces?
(Essay)
4.9/5  (32)
(32)
The boiling point of a liquid is the temperature at which the vapor pressure and atmospheric pressure are the same.
(True/False)
4.9/5  (36)
(36)
A 5.00 liter gas sample is collected at a temperature and pressure of 27.0 ° C and 1.20 atm. It is desired to transfer the gas to a 3.00 liter container at a pressure of 1.00 atm. What must be the temperature of the gas in the 3.00 liter container?
(Multiple Choice)
4.8/5  (36)
(36)
At STP, the vapor pressure of CH3CH2CH2CH2CH3 is less than CH3CH2CH2CH3.
(True/False)
5.0/5  (24)
(24)
What pressure will 3.20 mol of N2 gas exert if confined in a 15.0 L container at 100 ° C?
(Multiple Choice)
4.9/5  (41)
(41)
Consider the figure below which shows the compression phase of a piston in your car's engine. This is an example of which gas law?
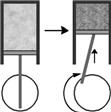
(Multiple Choice)
4.8/5  (42)
(42)
A 3.2 liter sample of gas is at 40 ° C and 1.0 atmosphere of pressure. If the temperature decreases to 20 ° C and the pressure decreases to 0.60 atmospheres, what is the new volume in liters?
(Multiple Choice)
4.9/5  (36)
(36)
How many balloons can you fill from a 10 liter tank of helium you purchased? The tank has an initial pressure of 1500 psi. Also, you know that, when filled to the recommended 25 psi, each balloon will hold a volume of 5.5 L. (Hint: Don't forget that you can't remove the last 10 L from the tank.)
(Multiple Choice)
4.8/5  (34)
(34)
Near room temperature air has a density of 1.18 × 10-3 g\mL. What volume will 30.77 g of air occupy?
(Multiple Choice)
4.8/5  (34)
(34)
Pure substances in the gaseous state contain more energy than in the liquid state.
(True/False)
4.9/5  (47)
(47)
Cohesive forces are the attractive forces associated with potential energy.
(True/False)
4.9/5  (30)
(30)
Polar liquids would normally have higher heats of vaporization than non-polar liquids.
(True/False)
4.8/5  (38)
(38)
Overtime, a latex balloon will lose its shape and buoyancy. Which process can be used to explain why this occurs?
(Multiple Choice)
4.7/5  (32)
(32)
A gas sample collected at 35.0°C and a pressure of 710 torr has a volume of 200 mL. The temperature and pressure are changed, and the gas is compressed to 150 mL. The new temperature is 40.0 ° C; what is the new pressure?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)