Exam 4: Forces Between Particles
Exam 1: Matter, Measurements, and Calculations101 Questions
Exam 2: Atoms and Molecules96 Questions
Exam 3: Electronic Structure and the Periodic Law91 Questions
Exam 4: Forces Between Particles94 Questions
Exam 5: Chemical Reactions95 Questions
Exam 6: The States of Matter95 Questions
Exam 7: Solutions and Colloids98 Questions
Exam 8: Reaction Rates and Equilibrium92 Questions
Exam 9: Acids, Bases, and Salts92 Questions
Exam 10: Radioactivity and Nuclear Processes93 Questions
Exam 11: Organic Compounds: Alkanes92 Questions
Exam 12: Unsaturated Hydrocarbons94 Questions
Exam 13: Alcohols, Phenols, and Ethers92 Questions
Exam 14: Aldehydes and Ketones93 Questions
Exam 15: Carboxylic Acids and Esters94 Questions
Exam 16: Amines and Amides92 Questions
Exam 17: Carbohydrates93 Questions
Exam 18: Lipids96 Questions
Exam 19: Proteins97 Questions
Exam 20: Enzymes94 Questions
Exam 21: Nucleic Acids and Protein Synthesis98 Questions
Exam 22: Nutrition and Energy for Life94 Questions
Exam 23: Carbohydrate Metabolism95 Questions
Exam 24: Lipid and Amino Acid Metabolism99 Questions
Exam 25: Body Fluids91 Questions
Select questions type
Which of the following has the highest electronegativity?
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
A
Which of the following would you expect to be the most polar molecule?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
B
A polyatomic ion is a species that has both ionic and covalent bonding.
(True/False)
5.0/5  (45)
(45)
Which of the following distinguishing electron configurations is characteristic of noble gases?
(Multiple Choice)
4.8/5  (32)
(32)
The reason that an overabundance of iron in the human body, hemochromatosis, is dangerous because iron is magnetic and the increase in magnetism in the body cause organ damage.
(True/False)
4.8/5  (44)
(44)
How many moles of K+ and P3- ions are present in 2.00 moles of K3P?
(Multiple Choice)
4.9/5  (41)
(41)
Bulimia is characterized as the failure to maintain a body mass that is at least 85% of normal.
(True/False)
4.9/5  (42)
(42)
Which of the following statements is correct with reference to covalent bonding?
(Multiple Choice)
4.8/5  (29)
(29)
The correct formula of an ionic compound containing Fe2+ and ClO 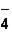 is _____ .
is _____ .
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following would be considered as a rare earth?
(Multiple Choice)
5.0/5  (37)
(37)
In what part of the human body is nitric oxide, NO, produced?
(Multiple Choice)
4.8/5  (29)
(29)
If the electronegativity difference between A and B is 0.8, what type of bond is formed between the two elements?
(Multiple Choice)
4.7/5  (28)
(28)
What single condition is required to insure that a molecule will be polar covalent?
(Multiple Choice)
4.8/5  (33)
(33)
In describing the strength of interparticle forces, we discover that the weakest forces of bonds are _____ .
(Multiple Choice)
4.8/5  (27)
(27)
Hydrogen bonds form between the molecules of the hypothetical compound ABCH and do not form between DEFH molecules.
(Multiple Choice)
4.9/5  (39)
(39)
What is the formula mass of the compound formed between Al3+ and Cl-?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)