Exam 2: The Chemists Toolbox
Exam 1: Molecular Reasons72 Questions
Exam 2: The Chemists Toolbox78 Questions
Exam 3: Atoms and Elements88 Questions
Exam 4: Molecules, Compounds, and Chemical Reactions83 Questions
Exam 5: Chemical Bonding79 Questions
Exam 6: Organic Chemistry76 Questions
Exam 7: Light and Color66 Questions
Exam 8: Nuclear Chemistry73 Questions
Exam 9: Energy for Today72 Questions
Exam 10: Energy for Tomorrow: Solar and Other Renewable Energy Sources70 Questions
Exam 11: The Air Around Us72 Questions
Exam 12: The Liquids and Solids Around Us: Especially Water75 Questions
Exam 13: Acids and Bases: the Molecules Responsible for Sour and Bitter74 Questions
Exam 14: Oxidation and Reduction71 Questions
Exam 15: The Chemistry of Household Products68 Questions
Exam 16: Biochemistry and Biotechnology71 Questions
Exam 17: Drugs and Medicine: Healing, Helping, and Hurting70 Questions
Exam 18: The Chemistry of Food66 Questions
Exam 19: Nanotechnology Online Only33 Questions
Select questions type
Which of the following is correctly written using scientific notation?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Determine the volume in liters of a 1.00-ounce bottle.(1.06 qt = 1 L; 32 ounces = 1 qt)
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
Figure 2-1 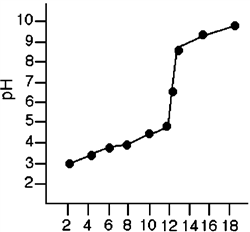 Refer to Figure 2-1. What is the pH of the solution after 8 mL of the base has been added?
Refer to Figure 2-1. What is the pH of the solution after 8 mL of the base has been added?
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
B
The long jump record is 8.90 m. What is the length in inches? (1 m = 39.37 in )
(Multiple Choice)
4.9/5  (34)
(34)
Which of these is the correct scientific notation for 6,000,220?
(Multiple Choice)
5.0/5  (38)
(38)
The density of gold is 19.3 g/mL. If the current price of gold is $56.75 per gram, what is the volume of a nugget of gold worth $150.00?
(Multiple Choice)
4.9/5  (37)
(37)
Which of these samples of aluminum will occupy the greatest volume? (Density of aluminum = 2.70 g/cm3; 454 g = 1 pound)
(Multiple Choice)
4.8/5  (45)
(45)
How many kilograms of calcium are there in 173 lb of calcium? (1 lb = 454 g)
(Multiple Choice)
4.9/5  (36)
(36)
The result of a multiplication or division should carry the same number of significant digits as the factor with the _____.
(Multiple Choice)
4.8/5  (24)
(24)
Which of these samples of water will have the greatest mass? (Density of water = 1.00 g/cm3; 454 g = 1 pound)
(Multiple Choice)
4.8/5  (34)
(34)
Figure 2-1 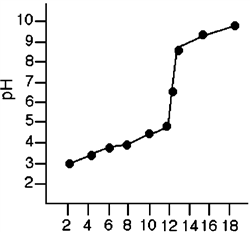 Refer to Figure 2-1. What was the effect on the pH of the solution when the volume of the base was increased from 8 mL to 13 mL?
Refer to Figure 2-1. What was the effect on the pH of the solution when the volume of the base was increased from 8 mL to 13 mL?
(Multiple Choice)
4.8/5  (37)
(37)
An irregularly shaped piece of metal with a mass of 105 g was placed in a graduated cylinder that contained 25.00 mL of water. This raised the water level to 45.35 mL. What is the density of the metal?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 1 - 20 of 78
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)