Exam 4: Molecules, Compounds, and Chemical Reactions
Exam 1: Molecular Reasons72 Questions
Exam 2: The Chemists Toolbox78 Questions
Exam 3: Atoms and Elements88 Questions
Exam 4: Molecules, Compounds, and Chemical Reactions83 Questions
Exam 5: Chemical Bonding79 Questions
Exam 6: Organic Chemistry76 Questions
Exam 7: Light and Color66 Questions
Exam 8: Nuclear Chemistry73 Questions
Exam 9: Energy for Today72 Questions
Exam 10: Energy for Tomorrow: Solar and Other Renewable Energy Sources70 Questions
Exam 11: The Air Around Us72 Questions
Exam 12: The Liquids and Solids Around Us: Especially Water75 Questions
Exam 13: Acids and Bases: the Molecules Responsible for Sour and Bitter74 Questions
Exam 14: Oxidation and Reduction71 Questions
Exam 15: The Chemistry of Household Products68 Questions
Exam 16: Biochemistry and Biotechnology71 Questions
Exam 17: Drugs and Medicine: Healing, Helping, and Hurting70 Questions
Exam 18: The Chemistry of Food66 Questions
Exam 19: Nanotechnology Online Only33 Questions
Select questions type
Which of these is not a covalent compound?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
Which of these is the correct formula for potassium phosphate?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Using the old English measurements of volume listed below, determine the number of gallons in two gills. 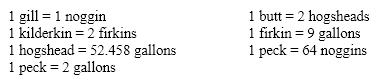
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
C
An international group of zookeepers with successful breeding programs made the following animal exchanges last year. Using the same bartering system, how many anteaters can a zoo obtain in exchange for 4 oryxes? 
(Multiple Choice)
4.8/5  (40)
(40)
How many moles of tetraphosphorous decaoxide will form when 3.30 moles of P 4 react with oxygen gas in the given equation?
P4 + 5 O2 → P4O10
(Multiple Choice)
4.9/5  (34)
(34)
How many moles of calcium atoms are there in 2.5 moles of calcium carbonate, CaCO3?
(Multiple Choice)
4.9/5  (34)
(34)
Automotive airbags inflate when a sample of NaN 3 (molar mass = 65 g/mol) is rapidly decomposed. What mass of NaN 3 is required to produce 368 L of nitrogen gas (molar mass = 28 g/mol) with a density of 1.25 g/L?
2 NaN3(s) → 2 Na(s) + 3 N2(g)
(Multiple Choice)
4.9/5  (42)
(42)
Using the old English measurements of volume listed below, determine the number of noggins in one butt. 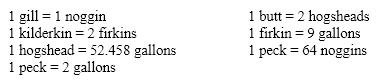
(Multiple Choice)
4.8/5  (36)
(36)
How many grams of water are produced when 10.0 grams of O2 react with excess H2?
2 H2 + O2(g) → 2 H2O
(Multiple Choice)
4.8/5  (32)
(32)
What is the mass in grams of 2.35 moles of calcium carbonate, CaCO3?
(Multiple Choice)
4.8/5  (35)
(35)
Using the old English measurements of volume listed below, determine the number of hogsheads in five kilderkins. 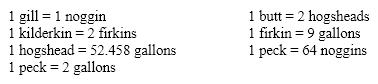
(Multiple Choice)
4.7/5  (47)
(47)
How many moles of carbon atoms are there in 5 moles of ethanol (CH3CH2OH)?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 1 - 20 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)