Exam 4: Molecules, Compounds, and Chemical Reactions
Exam 1: Molecular Reasons72 Questions
Exam 2: The Chemists Toolbox78 Questions
Exam 3: Atoms and Elements88 Questions
Exam 4: Molecules, Compounds, and Chemical Reactions83 Questions
Exam 5: Chemical Bonding79 Questions
Exam 6: Organic Chemistry76 Questions
Exam 7: Light and Color66 Questions
Exam 8: Nuclear Chemistry73 Questions
Exam 9: Energy for Today72 Questions
Exam 10: Energy for Tomorrow: Solar and Other Renewable Energy Sources70 Questions
Exam 11: The Air Around Us72 Questions
Exam 12: The Liquids and Solids Around Us: Especially Water75 Questions
Exam 13: Acids and Bases: the Molecules Responsible for Sour and Bitter74 Questions
Exam 14: Oxidation and Reduction71 Questions
Exam 15: The Chemistry of Household Products68 Questions
Exam 16: Biochemistry and Biotechnology71 Questions
Exam 17: Drugs and Medicine: Healing, Helping, and Hurting70 Questions
Exam 18: The Chemistry of Food66 Questions
Exam 19: Nanotechnology Online Only33 Questions
Select questions type
Metals tend to _____ electrons and form ions with a _____ charge.
(Multiple Choice)
4.7/5  (39)
(39)
What is the coefficient of iron oxide when the equation is balanced?
_____ Fe2O3 + _____ C → _____ Fe + _____ CO2
(Multiple Choice)
4.8/5  (30)
(30)
In the following equation, what is the coefficient of HF when the equation is balanced?
_____ B 2 O 3 + _____ HF → _____ BF 3 + _____ H 2 O
(Multiple Choice)
4.9/5  (38)
(38)
Consider the reaction: 4 NH3 + 5 O2 → 6 H2O + 4 NO
If 125.0 g of NH3 reacts with 225.0 g of O2, how many grams of H2O will be present after a complete reaction?
(Multiple Choice)
4.7/5  (36)
(36)
Nonmetals tend to _____ electrons and form ions with a _____ charge.
(Multiple Choice)
4.9/5  (40)
(40)
An international group of zookeepers with successful breeding programs made the following animal exchanges last year. Using the same bartering system, how many monkeys can a zoo obtain in exchange for 10 camels? 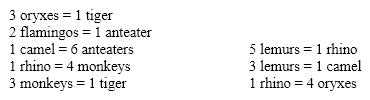
(Multiple Choice)
4.8/5  (26)
(26)
Identify a true statement about the naming of binary molecular compounds.
(Multiple Choice)
4.8/5  (40)
(40)
Nicotine has the chemical formula C10H14N2. What is the formula mass of nicotine?
(Multiple Choice)
5.0/5  (42)
(42)
In the following equation, what is the coefficient of oxygen (O 2 ) when the equation is balanced?
_____ C 3 H 8 + _____ O 2 → _____ CO 2 + _____ H 2 O
(Multiple Choice)
4.9/5  (36)
(36)
An international group of zookeepers with successful breeding programs made the following animal exchanges last year. Using the same bartering system, how many flamingos can a zoo obtain in exchange for 6 rhinos? 
(Multiple Choice)
4.8/5  (29)
(29)
How many moles of potassium cyanide are present in a 113.5-gram sample of KCN?
(Multiple Choice)
4.8/5  (37)
(37)
When properly balanced, what are the correct coefficients for the reaction H2 + O2 → H2O?
(Multiple Choice)
4.8/5  (40)
(40)
How many atoms of aluminum are contained in a 36.0-ounce aluminum bat? (1 ounce = 28.35 grams)
(Multiple Choice)
4.8/5  (33)
(33)
For an ionic compound, X 2 Y 5 , if the charge on each Y ion is − 2, the charge on each X ion is _____.
(Multiple Choice)
4.8/5  (41)
(41)
Which of these is the correct chemical formula for carbon tetrachloride?
(Multiple Choice)
4.8/5  (33)
(33)
Consider the reaction: 4 NH3 + 5 O2 → 6 H2O + 4 NO
If 2.5 moles of NH3 react with 3.0 moles of O2, what is the limiting reagent?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 21 - 40 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)