Exam 4: Logarithmic Functions and Exponential Modela
Exam 1: Data, Functions, and Models81 Questions
Exam 2: Linear Functions and Models70 Questions
Exam 3: Exponential Functions and Models110 Questions
Exam 4: Logarithmic Functions and Exponential Modela74 Questions
Exam 5: Quadratic Functions and Models73 Questions
Exam 6: Power, Polynomial, and Rational Functions71 Questions
Exam 7: Systems of Equations and Data in Categories71 Questions
Select questions type
Solve the exponential equation  for
for  . Round to the nearest thousandth.
. Round to the nearest thousandth.
(Multiple Choice)
4.7/5  (42)
(42)
Find the common logarithm of the given value, rounded to the nearest thousandth, of:
The weight of an African Grey parrot: 380 grams.
(Multiple Choice)
4.7/5  (35)
(35)
Solve the logarithmic equation  for
for  . Round to the nearest hundredth.
. Round to the nearest hundredth.
(Multiple Choice)
5.0/5  (39)
(39)
Which one of the following is the correct expansion of the logarithm expression  ?
?
(Multiple Choice)
4.9/5  (38)
(38)
Using the graph below, determine which one of the following exponential functions models the graph. 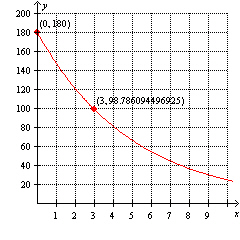
(Multiple Choice)
4.9/5  (32)
(32)
If $18,000 is invested at an interest rate of 4.5% compounded continuously, what is the amount of the investment after 10 years? Rounded to the nearest hundredth.
(Multiple Choice)
4.8/5  (32)
(32)
Which one of the following is the correct expansion of the logarithm expression  ?
?
(Multiple Choice)
4.8/5  (31)
(31)
Use the Change of Base Formula to evaluate the logarithm 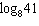 . Round to the nearest ten-thousandth.
. Round to the nearest ten-thousandth.
(Multiple Choice)
4.9/5  (32)
(32)
Two functions and are given by the tables below. Determine . 
(Multiple Choice)
4.9/5  (33)
(33)
Which one of the following is the correct expansion of the logarithm expression  ?
?
(Multiple Choice)
4.8/5  (37)
(37)
If we know the amount of light that is absorbed in a solution, we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  , where
, where  is the intensity of the incident light and
is the intensity of the incident light and  is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity  is 45% of
is 45% of  ?
?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 61 - 74 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)