Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control
Exam 1: Biochemistry and the Organization of Cells73 Questions
Exam 2: Water: the Solvent for Biochemical Reactions85 Questions
Exam 3: Amino Acids and Peptides75 Questions
Exam 4: The Three-Dimensional Structure of Proteins82 Questions
Exam 5: Protein Purification and Characterization Techniques67 Questions
Exam 6: The Behavior of Proteins: Enzymes83 Questions
Exam 7: The Behavior of Proteins: Enzymes, Mechanisms, and Control81 Questions
Exam 8: Lipids and Proteins Are Associated in Biological Membranes90 Questions
Exam 9: Nucleic Acids: How Structure Conveys Information66 Questions
Exam 10: Biosynthesis of Nucleic Acids: Replication86 Questions
Exam 11: Transcription of the Genetic Code: Biosynthesis of RNA98 Questions
Exam 12: Protein Synthesis: Translation of the Genetic Message85 Questions
Exam 13: Nucleic Acid Biotechnology Techniques94 Questions
Exam 14: Viruses, Cancer, and Immunology42 Questions
Exam 15: The Importance of Energy Changes and Electron Transfer in Metabolism60 Questions
Exam 16: Carbohydrates92 Questions
Exam 17: Glycolysis67 Questions
Exam 18: Storage Mechanisms and Control in Carbohydrate Metabolism82 Questions
Exam 19: The Citric Acid Cycle80 Questions
Exam 20: Electron Transport and Oxidative Phosphorylation66 Questions
Exam 21: Lipid Metabolism95 Questions
Exam 22: Photosynthesis74 Questions
Exam 23: The Metabolism of Nitrogen78 Questions
Exam 24: Integration of Metabolism: Cellular Signaling68 Questions
Select questions type
ATP is a negative allosteric effector for glycogen phosporylase. This is an example of
(Multiple Choice)
4.7/5  (37)
(37)
The main distinguishing feature of the concerted model for the behavior of allosteric enzymes is that
(Multiple Choice)
4.8/5  (34)
(34)
The pH profile of an enzyme can help identify specific amino acids in the active site because:
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following best describes negative cooperativity?
(Multiple Choice)
4.9/5  (40)
(40)
Enzymes that catalyze similar functions will invariably have
(Multiple Choice)
4.8/5  (33)
(33)
An important step in elucidating the behavior of an enzyme is
(Multiple Choice)
4.9/5  (48)
(48)
Which groups of amino acids are most likely to be found in the active site of an enzyme?
(Multiple Choice)
4.9/5  (35)
(35)
According to the concerted model of allosteric behavior, an allosteric activator
(Multiple Choice)
4.9/5  (34)
(34)
A velocity curve (V vs. [S]) for a typical allosteric enzyme will be
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following types of amino acids in an active site is least likely to be involved in enzyme catalysis?
(Multiple Choice)
4.8/5  (42)
(42)
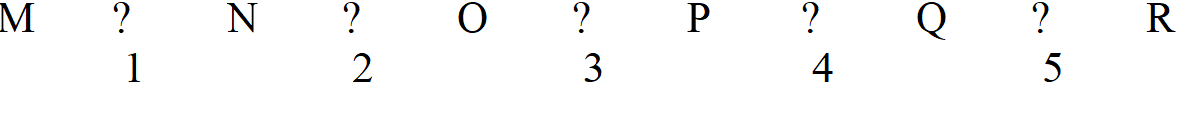 -Refer to Exhibit 7A. Which two enzymes would be the most likely ones to regulate if this pathway is freely reversible and can go both ways?
-Refer to Exhibit 7A. Which two enzymes would be the most likely ones to regulate if this pathway is freely reversible and can go both ways?
(Multiple Choice)
5.0/5  (37)
(37)
Is the Michaelis-Menten equation useful when studying allosteric enzymes?
(Multiple Choice)
4.8/5  (33)
(33)
Metal ions play an important role in reaction mechanisms because
(Multiple Choice)
4.9/5  (37)
(37)
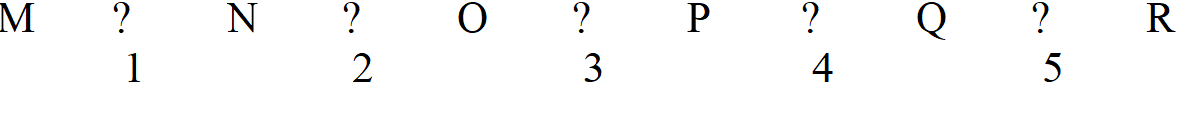 -Refer to Exhibit 7A. The final product, R, will most likely inhibit which reaction?
-Refer to Exhibit 7A. The final product, R, will most likely inhibit which reaction?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 61 - 80 of 81
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)