Exam 15: Acids and Bases
Exam 1: Units of Measurement for Physical and Chemical Change179 Questions
Exam 2: Atoms and Elements167 Questions
Exam 3: Molecules, Compounds, and Nomenclature178 Questions
Exam 4: Chemical Reactions and Stoichiometry230 Questions
Exam 5: Gases154 Questions
Exam 6: Thermochemistry156 Questions
Exam 7: The Quantum-Mechanical Model of the Atom173 Questions
Exam 8: Periodic Properties of the Elements127 Questions
Exam 9: Chemical Bonding I: Lewis Theory143 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory168 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces132 Questions
Exam 12: Solutions159 Questions
Exam 13: Chemical Kinetics162 Questions
Exam 14: Chemical Equilibrium123 Questions
Exam 15: Acids and Bases148 Questions
Exam 16: Aqueous Ionic Equilibrium161 Questions
Exam 17: Gibbs Energy and Thermodynamics111 Questions
Exam 18: Electrochemistry126 Questions
Exam 19: Radioactivity and Nuclear Chemistry115 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry II: Reactions96 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallury49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
What is the selenide ion concentration [Se2-] for a 0.100 mol L-1 H2Se solution that has the stepwise dissociation constants of Ka1 = 1.3 × 10-4 and Ka2 = 1.0 × 10-11?
(Multiple Choice)
4.8/5  (33)
(33)
Determine the Ka for CH3NH3⁺ at 25 °C. The Kb for CH3NH2 is 4.4 × 10-4.
(Multiple Choice)
4.8/5  (29)
(29)
Determine the pH of a 0.188 mol L-1 NH3 solution at 25 °C. The Kb of NH3 is 1.76 × 10-5.
(Multiple Choice)
4.9/5  (35)
(35)
Identify the Lewis acid, Lewis base, and Lewis acid-base adduct in the following equation: (C2H5)2O + BF3 ⇌ (C2H5)2O:BF3
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following bases is the strongest? The base is followed by its Kb.
(Multiple Choice)
4.8/5  (43)
(43)
The acid-dissociation constant at 25.0 °C for hypochlorous acid (HClO)is 3.0 × 10-8. At equilibrium, the molarity of H3O+ in a 0.010 mol L-1 solution of HClO is ________.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following equilibria represents the second ionization step of H3PO4?
(Multiple Choice)
4.9/5  (32)
(32)
Determine the [OH⁻] concentration in a 0.169 mol L-1 Ca(OH)2 solution.
(Multiple Choice)
4.9/5  (30)
(30)
Calculate the pH of a 1.60 mol L-1 KBrO solution. Ka for hypobromous acid, HBrO, is 2.0 × 10-9.
(Multiple Choice)
4.9/5  (38)
(38)
Determine the [OH-] concentration of a 0.116 mol L-1 Ba(OH)2 solution at 25 °C.
(Multiple Choice)
4.9/5  (35)
(35)
A 7.0 × 10-3 mol L-1 aqueous solution of Ca(OH)2 at 25.0 °C has a pH of ________.
(Multiple Choice)
4.9/5  (31)
(31)
What is the hydronium ion concentration of a 0.500 mol L-1 acetic acid solution with Ka = 1.8 × 10-5? The equation for the dissociation of acetic acid is below: 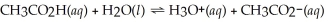
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the pH for an aqueous solution of acetic acid that contains 2.15 × 10-3 mol L-1 hydronium ion.
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the hydroxide ion concentration in an aqueous solution with a pH of 4.33 at 25 °C.
(Multiple Choice)
4.9/5  (33)
(33)
Which one of the following will form a basic solution in water?
(Multiple Choice)
4.9/5  (44)
(44)
Showing 21 - 40 of 148
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)