Exam 8: Water Everywhere: A Most Precious Resource
Exam 1: Portable Electronics: the Periodic Table in the Palm of Your Hand50 Questions
Exam 2: The Air We Breathe50 Questions
Exam 3: Radiation From the Sun50 Questions
Exam 4: Climate Change8 Questions
Exam 5: Energy From Combustion5 Questions
Exam 6: Energy From Alternative Sources50 Questions
Exam 7: Energy Storage18 Questions
Exam 8: Water Everywhere: A Most Precious Resource79 Questions
Exam 9: The World of Polymers and Plastics72 Questions
Exam 10: Brewing and Chewing56 Questions
Exam 11: Nutrition47 Questions
Exam 12: Health Medicine47 Questions
Exam 13: Genes and Life48 Questions
Exam 14: Who Killed Drthompson a Forensic Mystery50 Questions
Select questions type
A student wants to prepare exactly 250 mL of a 0.500 M aqueous potassium hydroxide solution.What mass of potassium hydroxide (molar mass = 56.10 g/mol) must the student dissolve in the 250 mL of solution?
(Multiple Choice)
4.7/5  (39)
(39)
The drawing shows a simple way of purifying salt water.What is this process called? 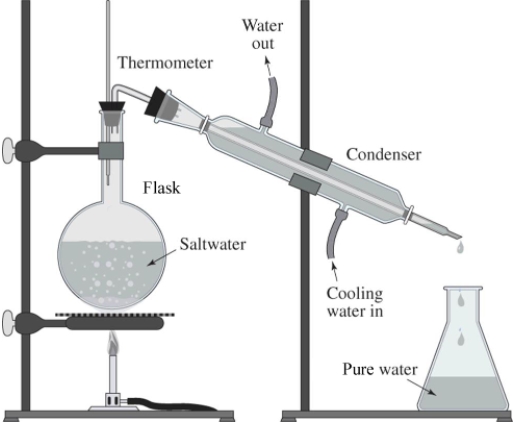
(Multiple Choice)
4.9/5  (42)
(42)
If the lead concentration in water is 1 ppm,then we should be able to recover 1 mg of lead from _____ L of water.
(Multiple Choice)
4.7/5  (46)
(46)
An acidic fog in Pasadena was found to have a pH of 2.50.Which expression represents this pH measurement?
(Multiple Choice)
4.7/5  (38)
(38)
At water treatment plants aluminum sulfate and calcium hydroxide are added to
(Multiple Choice)
4.7/5  (44)
(44)
What is the concentration of hydroxide ions in an aqueous solution containing [H+] = 1 x 10-5 M?
(Multiple Choice)
4.9/5  (38)
(38)
We cannot effectively clean nonpolar substances from our hands or clothing with water alone;we must add soap or detergent.The structure of a typical soap molecule is shown below.Which region of this molecule would dissolve in a nonpolar substance such as grease? 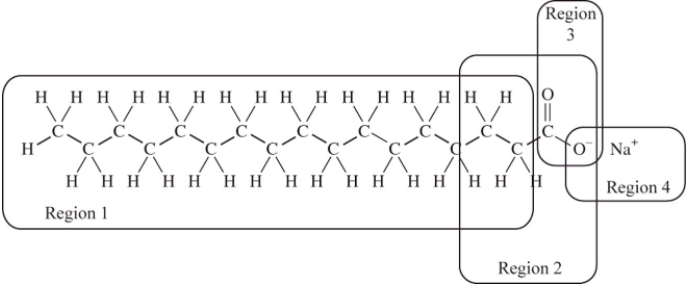
(Multiple Choice)
4.8/5  (33)
(33)
Which shows the Lewis structure of water with the correct partial charges and nonbonding electrons?
(Multiple Choice)
4.8/5  (45)
(45)
How many moles of sodium chloride are there in 250 mL of a 1.20 M sodium chloride solution?
(Multiple Choice)
4.8/5  (31)
(31)
Which molecule(s) contain(s) polar covalent bonds,but is(are) nonpolar? 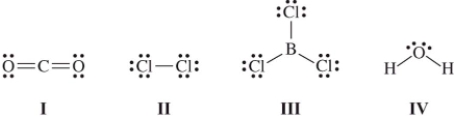
(Multiple Choice)
4.9/5  (32)
(32)
Mixing which of the following will produce a precipitation reaction (give an insoluble product)?
(Multiple Choice)
4.7/5  (42)
(42)
Which chemical equation shows the dissociation of two protons from trihydrogen phosphate (phosphoric acid)?
(Multiple Choice)
4.9/5  (38)
(38)
The attractions between anions and cations throughout a crystal are known collectively as
(Multiple Choice)
4.9/5  (31)
(31)
How many joules are required to heat 2.0 L of water from 20 C to its boiling point of 100 C? The specific heat of water is 4.18 J/g. C and the density of water is 1 g/mL.
(Multiple Choice)
4.7/5  (31)
(31)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)