Exam 8: Water Everywhere: A Most Precious Resource
Exam 1: Portable Electronics: the Periodic Table in the Palm of Your Hand50 Questions
Exam 2: The Air We Breathe50 Questions
Exam 3: Radiation From the Sun50 Questions
Exam 4: Climate Change8 Questions
Exam 5: Energy From Combustion5 Questions
Exam 6: Energy From Alternative Sources50 Questions
Exam 7: Energy Storage18 Questions
Exam 8: Water Everywhere: A Most Precious Resource79 Questions
Exam 9: The World of Polymers and Plastics72 Questions
Exam 10: Brewing and Chewing56 Questions
Exam 11: Nutrition47 Questions
Exam 12: Health Medicine47 Questions
Exam 13: Genes and Life48 Questions
Exam 14: Who Killed Drthompson a Forensic Mystery50 Questions
Select questions type
Hard water in parts of the Midwest may have a calcium ion concentration as high as 400 ppm.What is this calcium ion concentration when expressed as a percentage?
(Multiple Choice)
4.9/5  (38)
(38)
The pH of rain water falling through an unpolluted atmosphere is closest to
(Multiple Choice)
5.0/5  (52)
(52)
The drawing shows two water molecules.Which statement is correct? 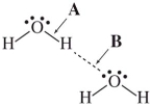
(Multiple Choice)
4.8/5  (23)
(23)
Calculate the pH of a solution prepared by dissolving 1.2 g of potassium hydroxide (KOH) in 1,250 mL of water.
(Multiple Choice)
4.8/5  (39)
(39)
Reverse osmosis is illustrated in this diagram.What is this process used for? 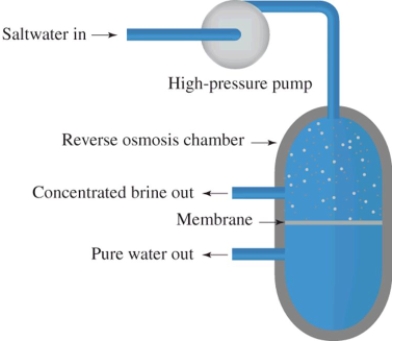
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following are methods of making sea water appropriate for drinking? (More than one choice may be appropriate. )
(Multiple Choice)
4.8/5  (40)
(40)
Numbers 1 through 4 are used to identify four different elements.Based on periodic table trends,which number identifies the element that is expected to have the greatest tendency to attract a shared pair of electrons? 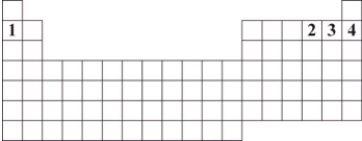
(Multiple Choice)
4.8/5  (37)
(37)
What atmospheric component is responsible for the natural acidity of rain?
(Multiple Choice)
4.8/5  (36)
(36)
A proton released by an acid in aqueous solution quickly reacts with water to form a hydronium ion.What product is formed when a proton reacts with ammonia (NH3)?
(Multiple Choice)
4.9/5  (36)
(36)
The fact that carbon (C) is less electronegative than nitrogen (N) means that in a C - N bond,the
(Multiple Choice)
4.8/5  (34)
(34)
A 0.25 M aqueous solution of potassium chloride,KCl,is tested for conductivity using the type of apparatus shown.What do you predict will happen? 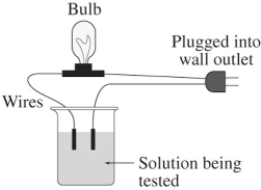
(Multiple Choice)
4.7/5  (29)
(29)
Predict the products of the chemical equation: 3 LiOH + H3PO4
(Multiple Choice)
4.9/5  (35)
(35)
Lakes surrounded by ________ have very little acid-neutralizing capacity. I.marble
II)granite
III)limestone
(Multiple Choice)
4.9/5  (40)
(40)
Which reaction represents an acid-base neutralization reaction?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 21 - 40 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)