Exam 8: Water Everywhere: A Most Precious Resource
Exam 1: Portable Electronics: the Periodic Table in the Palm of Your Hand50 Questions
Exam 2: The Air We Breathe50 Questions
Exam 3: Radiation From the Sun50 Questions
Exam 4: Climate Change8 Questions
Exam 5: Energy From Combustion5 Questions
Exam 6: Energy From Alternative Sources50 Questions
Exam 7: Energy Storage18 Questions
Exam 8: Water Everywhere: A Most Precious Resource79 Questions
Exam 9: The World of Polymers and Plastics72 Questions
Exam 10: Brewing and Chewing56 Questions
Exam 11: Nutrition47 Questions
Exam 12: Health Medicine47 Questions
Exam 13: Genes and Life48 Questions
Exam 14: Who Killed Drthompson a Forensic Mystery50 Questions
Select questions type
The recommended daily requirement of calcium is 1,000 mg.Tap water in a Midwestern city contains approximately 150 mg Ca/L.A person living in the city who drinks two liters of water in one day would receive ________ percent of his/her RDA of calcium.
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
D
What is the molarity of sodium chloride in a solution containing 0.50 mol of sodium chloride in 500 mL of water?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
When table sugar (sucrose,C12H22O11) dissolves in water,which type(s) of attraction between water and sugar molecules occur(s)? I.hydrogen bonding
II)polar-polar interactions
III)covalent bonding
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
A
Which chemical equation shows the dissociation of magnesium hydroxide?
(Multiple Choice)
4.8/5  (35)
(35)
Most aquatic life in lakes cannot survive in water with a pH less than
(Multiple Choice)
5.0/5  (44)
(44)
Which of the following is not a consequence of biomagnification?
(Multiple Choice)
4.8/5  (33)
(33)
A 0.25 M solution of the sugar sucrose,C12H22O11,in water is tested for conductivity using the type of apparatus shown.What do you predict will happen? 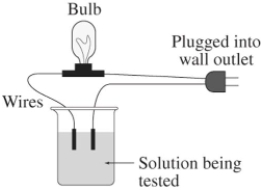
(Multiple Choice)
4.7/5  (35)
(35)
What are the major disadvantages of using ozone instead of chlorine to disinfect water?
(Multiple Choice)
4.7/5  (24)
(24)
Which is not a consequence of hydrogen bonding between water molecules?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following is not carried out by bacteria in soil?
(Multiple Choice)
4.7/5  (39)
(39)
The acid neutralizing capacity of a lake or stream most often derives from the presence of ________ in the surrounding soil or rock.
(Multiple Choice)
4.8/5  (37)
(37)
Which form of water disinfection continues to provide antibacterial protection after the water leaves the purification plant?
(Multiple Choice)
4.8/5  (43)
(43)
Which reaction accounts for the fact that the pH of rain is naturally slightly acidic?
(Multiple Choice)
4.9/5  (35)
(35)
Which reaction most accurately represents the dissociation of nitrous acid (HNO2) in water?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)