Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Matter, Measurement, and Problem Solving120 Questions
Exam 2: Atoms and Elements116 Questions
Exam 3: Molecules, Compounds and Chemical Equations144 Questions
Exam 4: Chemical Quantities and Aqueous Reactions199 Questions
Exam 5: Gases157 Questions
Exam 6: Thermochemistry110 Questions
Exam 7: The Quantum-Mechanical Model of the Atom100 Questions
Exam 8: Periodic Properties of the Elements120 Questions
Exam 9: Chemical Bonding I: Lewis Theory125 Questions
Exam 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and109 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces123 Questions
Exam 12: Solutions127 Questions
Exam 13: Chemical Kinetics125 Questions
Exam 14: Chemical Equilibrium112 Questions
Exam 15: Acids and Bases126 Questions
Exam 16: Aqueous Ionic Equilibrium148 Questions
Exam 17: Free Energy and Thermodynamics103 Questions
Exam 18: Electrochemistry115 Questions
Exam 19: Radioactivity and Nuclear Chemistry105 Questions
Select questions type
Identify the compound that does not have dipole-dipole forces as its strongest force.
(Multiple Choice)
5.0/5  (35)
(35)
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
(Multiple Choice)
4.8/5  (28)
(28)
Place the following substances in order of increasing boiling point.
Ne Cl2 O2
(Multiple Choice)
4.8/5  (35)
(35)
Determine ΔHvap for a compound that has a measured vapor pressure of 24.3 torr at 273 K and 135 torr at 325 K.
(Multiple Choice)
4.7/5  (32)
(32)
Ethyl chloride,C2H5Cl,is used as a local anesthetic.It works by cooling tissue as it vaporizes;its heat of vaporization is 26.4 kJ/mol.How much heat could be removed by 20.0 g of ethyl chloride?
(Multiple Choice)
4.9/5  (29)
(29)
A metal crystallizes in a face centered cubic structure and has a density of 11.9 g/cm3.If the radius of the metal atom is 138 pm,what is the identity of the metal?
(Multiple Choice)
4.9/5  (28)
(28)
Place the following substances in order of increasing vapor pressure at a given temperature.
SF6 SiH4 SF4
(Multiple Choice)
4.7/5  (37)
(37)
How many H- ions are around each Na+ ion in NaH,which has a cubic unit cell with H- ions on each corner and each face?
(Multiple Choice)
4.7/5  (37)
(37)
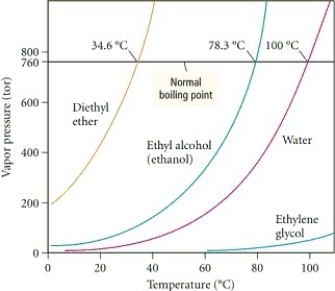 Based on the figure above,the boiling point of diethyl ether under an external pressure of 1.32 atm is approximately ________°C.
Based on the figure above,the boiling point of diethyl ether under an external pressure of 1.32 atm is approximately ________°C.
(Multiple Choice)
4.8/5  (37)
(37)
Place the following substances in order of decreasing vapor pressure at a given temperature.
BeF2 CH3OH OF2
(Multiple Choice)
4.8/5  (45)
(45)
Place the following substances in order of decreasing boiling point.
H2O N2 CO
(Multiple Choice)
4.7/5  (38)
(38)
What fraction of an atom is at each corner a face-centered cubic unit cell?
(Multiple Choice)
4.8/5  (32)
(32)
Determine the normal boiling point of a substance whose vapor pressure is 55.1 mm Hg at 35°C and has a ΔHvap of 32.1 kJ/mol.
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following substances should have the highest melting point?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following substances would you predict to have the highest ΔHvap?
(Multiple Choice)
4.8/5  (36)
(36)
Determine the vapor pressure (in torr)of a substance at 36°C,whose normal boiling point is 84°C and has a ΔHvap of 22.1 kJ/mol.
(Multiple Choice)
4.8/5  (40)
(40)
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH)at its boiling point,if its ΔHvap is 40.5 kJ/mol?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 101 - 120 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)