Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Matter, Measurement, and Problem Solving120 Questions
Exam 2: Atoms and Elements116 Questions
Exam 3: Molecules, Compounds and Chemical Equations144 Questions
Exam 4: Chemical Quantities and Aqueous Reactions199 Questions
Exam 5: Gases157 Questions
Exam 6: Thermochemistry110 Questions
Exam 7: The Quantum-Mechanical Model of the Atom100 Questions
Exam 8: Periodic Properties of the Elements120 Questions
Exam 9: Chemical Bonding I: Lewis Theory125 Questions
Exam 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and109 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces123 Questions
Exam 12: Solutions127 Questions
Exam 13: Chemical Kinetics125 Questions
Exam 14: Chemical Equilibrium112 Questions
Exam 15: Acids and Bases126 Questions
Exam 16: Aqueous Ionic Equilibrium148 Questions
Exam 17: Free Energy and Thermodynamics103 Questions
Exam 18: Electrochemistry115 Questions
Exam 19: Radioactivity and Nuclear Chemistry105 Questions
Select questions type
Gold crystallizes in a face-centered cubic structure.What is the edge length of the unit cell if the atomic radius of gold is 144 pm?
(Multiple Choice)
4.7/5  (43)
(43)
The fluorocarbon C2Cl3F3 has a normal boiling point of 47.6°C.The specific heats of C2Cl3F3(l)and C2Cl3F3(g)are 0.91 J/g∙K and 0.67 J/g∙K,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 50.0°C to the gas at 80.0°C is ________ kJ.
(Multiple Choice)
4.8/5  (36)
(36)
Choose the pair of substances that are most likely to form a homogeneous solution.
(Multiple Choice)
4.9/5  (39)
(39)
Place the following substances in order of increasing boiling point.
CH3CH2OH Ar CH3OCH3
(Multiple Choice)
4.7/5  (47)
(47)
How much energy is required to vaporize 158 g of butane (C4H10)at its boiling point,if its ΔHvap is 24.3 kJ/mol?
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the vapor pressure of methanol at 40.0°C from the data given below:
Enthalpy of vaporization (∆Hvap = 35.2 kJ/mol;boiling point = 64.6°C;
Vapor pressure at 64.6°C = 760 torr)
(Multiple Choice)
4.8/5  (34)
(34)
Cesium has a radius of 272 pm and crystallizes in a body-centered cubic structure.What is the edge length of the unit cell?
(Multiple Choice)
4.7/5  (39)
(39)
Choose the substance with the lowest vapor pressure at a given temperature.
(Multiple Choice)
4.9/5  (36)
(36)
What is the strongest type of intermolecular force present in NH2CH3?
(Multiple Choice)
4.9/5  (44)
(44)
Determine the radius of an Al atom (in pm)if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 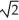 R.
R.
(Multiple Choice)
4.9/5  (37)
(37)
Why do O,F and N,when bonded to H,form such strong intermolecular attractions to neighboring molecules? Make sure to be specific.
(Essay)
4.8/5  (39)
(39)
Place the following compounds in order of increasing strength of intermolecular forces.
CH4 CH3CH2CH3 CH3CH3
(Multiple Choice)
5.0/5  (52)
(52)
The heat of vaporization of water at 100°C is 40.66 kJ/mol.Calculate the quantity of heat that is absorbed/released when 9.00 g of steam condenses to liquid water at 100°C.
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)