Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Matter, Measurement, and Problem Solving120 Questions
Exam 2: Atoms and Elements116 Questions
Exam 3: Molecules, Compounds and Chemical Equations144 Questions
Exam 4: Chemical Quantities and Aqueous Reactions199 Questions
Exam 5: Gases157 Questions
Exam 6: Thermochemistry110 Questions
Exam 7: The Quantum-Mechanical Model of the Atom100 Questions
Exam 8: Periodic Properties of the Elements120 Questions
Exam 9: Chemical Bonding I: Lewis Theory125 Questions
Exam 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and109 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces123 Questions
Exam 12: Solutions127 Questions
Exam 13: Chemical Kinetics125 Questions
Exam 14: Chemical Equilibrium112 Questions
Exam 15: Acids and Bases126 Questions
Exam 16: Aqueous Ionic Equilibrium148 Questions
Exam 17: Free Energy and Thermodynamics103 Questions
Exam 18: Electrochemistry115 Questions
Exam 19: Radioactivity and Nuclear Chemistry105 Questions
Select questions type
Ethanol (C2H5OH)melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g∙K and 2.3 J/g∙K,respectively.How much heat (kJ)is needed to convert 25.0 g of solid ethanol at -135°C to liquid ethanol at -50°C?
(Multiple Choice)
4.9/5  (39)
(39)
Explain why the bolling point of water is so much higher than other compounds of similar molecular weight.
(Essay)
4.9/5  (41)
(41)
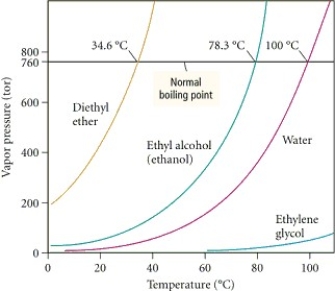 Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 0.0724 atm is approximately ________°C.
Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 0.0724 atm is approximately ________°C.
(Multiple Choice)
4.9/5  (46)
(46)
The enthalpy change for converting 1.00 mol of ice at -50.0°C to water at 70.0°C is  The specific heats of ice,water,and steam are
The specific heats of ice,water,and steam are
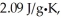
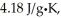 And
And
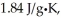 Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements is not correct regarding rate of vaporization of a liquid?
(Multiple Choice)
4.8/5  (29)
(29)
In the phase diagram,what is the approximate critical temperature? 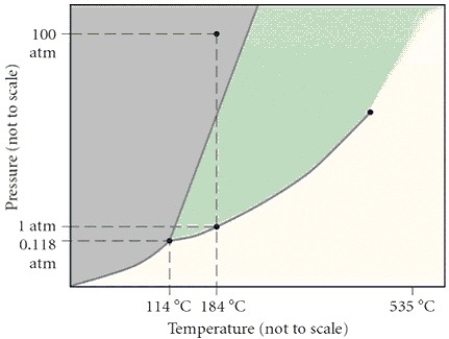
(Multiple Choice)
4.8/5  (37)
(37)
Identify the phase in which the water molecules are closest together.
(Multiple Choice)
4.8/5  (50)
(50)
Lithium crystallizes in a body-centered cubic structure.What is the coordination number of each atom?
(Multiple Choice)
4.7/5  (42)
(42)
If all of the following are in solid phase,which is considered a non-bonding atomic solid?
(Multiple Choice)
5.0/5  (37)
(37)
Describe the difference between the conduction band and the valence band.
(Essay)
4.8/5  (37)
(37)
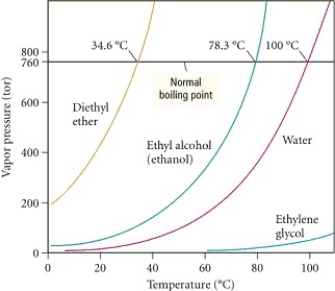 Based on the figure above,the boiling point of water under an external pressure of 0.316 atm is approximately ________°C.
Based on the figure above,the boiling point of water under an external pressure of 0.316 atm is approximately ________°C.
(Multiple Choice)
4.7/5  (35)
(35)
Showing 21 - 40 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)