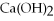Exam 10: Acids and Bases and Equilibrium
Exam 1: Chemistry in Our Lives42 Questions
Exam 2: Chemistry and Measurement83 Questions
Exam 3: Matter and Energy108 Questions
Exam 4: Atoms and Elements106 Questions
Exam 5: Nuclear Chemistry89 Questions
Exam 6: Ionic and Molecular Compounds103 Questions
Exam 7: Chemical Quantities and Reactions109 Questions
Exam 8: Gases73 Questions
Exam 9: Solutions78 Questions
Exam 10: Acids and Bases and Equilibrium65 Questions
Exam 11: Introduction to Organic Chemistry: Hydrocarbons132 Questions
Exam 12: Alcohols, Thiols, Ethers, Aldehydes, and Ketones83 Questions
Exam 13: Carbohydrates82 Questions
Exam 14: Carboxylic Acids, Esters, Amines, and Amides105 Questions
Exam 15: Lipids63 Questions
Exam 16: Amino Acids, Proteins, and Enzymes107 Questions
Exam 17: Nucleic Acids and Protein Synthesis59 Questions
Exam 18: Metabolic Pathways and Energy Production136 Questions
Select questions type
Identify the Brønsted-Lowry acid in the following reaction.
H2O(l)+ 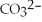 (aq)→
(aq)→ 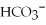 (aq)+
(aq)+ 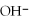 (aq)
(aq)
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
A chemical reaction has reached equilibrium when ________.
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
E
If the carbon dioxide level in the blood is too high,more carbonic acid is produced,and this results in the condition termed acidosis.
(True/False)
4.7/5  (39)
(39)
Which of the following is the correctly balanced equation for the complete neutralization of H3PO4 by Ca(OH)2 ?
(Multiple Choice)
4.8/5  (34)
(34)
An acid and base react to form a salt and water in a(n)________ reaction.
(Multiple Choice)
5.0/5  (28)
(28)
In the following solutions,is the [OH-] greater than,less than,or equal to the [H₃O+]?
Correct Answer:
Premises:
Responses:
(Matching)
5.0/5  (48)
(48)
What is the name of the medical condition of an asthmatic patient with a blood pH of 7.30?
(Multiple Choice)
4.8/5  (41)
(41)
According to the Arrhenius concept,if 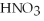 were dissolved in water,it would act as ________.
were dissolved in water,it would act as ________.
(Multiple Choice)
4.9/5  (32)
(32)
When hyperventilation (rapid breathing)causes a patient to exhale large amounts of 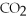 ,the blood pH rises in a condition called ________.
,the blood pH rises in a condition called ________.
(Multiple Choice)
4.7/5  (41)
(41)
Alkalosis is the blood condition in which the blood pH is higher than normal.
(True/False)
4.8/5  (29)
(29)
Which of the following statements correctly describes the hydronium-hydroxide balance in the given solution?
(Multiple Choice)
4.9/5  (35)
(35)
Identify the following as acids, bases, or neutral solutions.
Correct Answer:
Premises:
Responses:
(Matching)
5.0/5  (42)
(42)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)
![[ ] = 1.0 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_10d2_91e5_f9e4d804377e_TB6077_11.jpg)
![[ ] = 1.0 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_37e3_91e5_0f2bbb93c21e_TB6077_11.jpg) ] = 1.0 ×
] = 1.0 × ![[ ] = 1.0 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_37e4_91e5_9907b3bba0d0_TB6077_11.jpg) M
M![[ ] = 1 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_37e5_91e5_3b8c6bf379ae_TB6077_11.jpg)
![[ ] = 1 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_37e6_91e5_adf13cb8e0fc_TB6077_11.jpg) ] = 1 ×
] = 1 × ![[ ] = 1 × M](https://storage.examlex.com/TB6077/11ea71d0_de56_37e7_91e5_e1ea0a6f2f73_TB6077_11.jpg) M
M