Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Suppose a thermometer has marks at every one degree increment and the mercury level on the thermometer is exactly between the 25 and 26 degree Celsius marks.We should properly report the temperature measurement as:
(Multiple Choice)
4.8/5  (30)
(30)
Scientific numbers are reported so that every digit is certain except the last,which is estimated.
(True/False)
4.9/5  (40)
(40)
Determine the answer for the equation below with correct number of significant figures: 3.215 × 13.2 ÷ 0.218 = ________
(Multiple Choice)
4.7/5  (32)
(32)
The prefix in the name of a polygon indicates how many sides this geometric figure has,so a "decagon" would have ten sides.
(True/False)
4.7/5  (42)
(42)
The decimal number 0.0210 expressed in scientific notation is 2.10 × 10-2.
(True/False)
4.8/5  (41)
(41)
Conversion factors are constructed from any two quantities known to be equivalent.
(True/False)
5.0/5  (36)
(36)
Determine the answer to the following equation with correct number of significant figures: 2.02 + 8.102 - 0.0297 = ________
(Multiple Choice)
4.9/5  (36)
(36)
How would the number 8,155 be written in scientific notation?
(Multiple Choice)
4.9/5  (28)
(28)
How many significant figures should be reported in the answer to the following calculation? (43.980)× (19.0023 + 25)=
(Multiple Choice)
4.7/5  (40)
(40)
The density of iron is 7.86 g/cm3 while the density of lead is 11.4 g/cm3.If you had one cm3 of each metal,the piece of iron would have a greater mass.
(True/False)
4.8/5  (36)
(36)
The distance between the two hydrogen atoms in a molecule of water is 0.000000000172 m.Express this distance in scientific notation.
(Multiple Choice)
4.8/5  (32)
(32)
A 12-oz can of soda pop costs eighty-nine cents.A 2.00 L bottle of the same variety of soda pop costs $2.29.How many times more expensive it is to buy the 12-oz can of pop compared to buying it in a 2.00 L bottle? (1.00 L = 1.057 quart and 1 quart contains 32 oz)
(Multiple Choice)
4.8/5  (52)
(52)
When the number 2.35 is rounded to contain 2 significant figures it becomes 2.4.
(True/False)
4.9/5  (34)
(34)
How many significant figures should be reported in the answer to the following calculation? 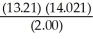 =
=
(Multiple Choice)
5.0/5  (34)
(34)
Which of the following would NOT be considered a correct conversion factor?
(Multiple Choice)
4.9/5  (35)
(35)
The Olympic Games shot put field event uses a 16 pound (lb)shot.Identify the correct solution map to convert from pounds to kilograms using prefix multipliers and the given conversions of 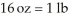 and
and 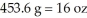 .
.
(Multiple Choice)
4.7/5  (41)
(41)
Showing 21 - 40 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)