Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Given the following list of densities,which materials would float in a molten vat of lead provided that they do not themselves melt? Densities (g/mL): lead = 11.4,glass = 2.6,gold = 19.3,charcoal = 0.57,platinum = 21.4.
(Multiple Choice)
4.8/5  (29)
(29)
A plastic block has dimensions of 2.2 cm × 3.0 cm × 1.5 cm and a mass of 12.4 grams.Will the block float in water and why?
(Multiple Choice)
4.9/5  (42)
(42)
The correct number of significant figures in the number 0.002320 is:
(Multiple Choice)
4.8/5  (32)
(32)
How many significant figures are represented by the following number that is written in scientific notation?
2.5 × 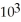
(Multiple Choice)
4.8/5  (39)
(39)
Determine the answer to the following equation with correct number of significant figures: 106 ÷ 9.02 × 1.9 = ________
(Multiple Choice)
4.7/5  (36)
(36)
The mass of an object,4.55 ×  g,expressed in decimal notation is 0.000455 g.
g,expressed in decimal notation is 0.000455 g.
(True/False)
4.7/5  (26)
(26)
How many significant figures should be reported in the answer to the following calculation? (8.50)× (29.0)× (1.0947)=
(Multiple Choice)
4.8/5  (35)
(35)
Zeros located after a number and after a decimal point are significant.
(True/False)
4.8/5  (36)
(36)
Given the density of Au is 19.3 g/cm3,determine the mass of gold (in grams)in an ingot with the dimensions of 10.0 in × 4.00 in × 3.00 in.
(Multiple Choice)
4.8/5  (43)
(43)
A conversion factor is a fraction with one unit on top and a different unit on the bottom.
(True/False)
4.7/5  (41)
(41)
There are exactly 2.54 centimeters in 1 inch.When using this conversion factor,how many significant figures are you limited to?
(Multiple Choice)
5.0/5  (38)
(38)
If you know the density of a liquid and its volume,the mass of the liquid may be calculated using the equation: m = V/d.
(True/False)
4.8/5  (46)
(46)
Showing 41 - 60 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)