Exam 2: Measurement and Problem Solving
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
The correct number of significant figures in the number 865,000 is:
(Multiple Choice)
4.9/5  (44)
(44)
When rounding the number 2.348615 to 4 significant figures,what is the correct value?
(Multiple Choice)
4.7/5  (34)
(34)
Determine the answer to the following equation with correct number of significant figures: (4.123 × 0.12)+ 24.2 = ________
(Multiple Choice)
4.8/5  (36)
(36)
Determine the answer to the following equation with correct number of significant figures: 13.96 - 4.9102 + 71.5 = ________
(Multiple Choice)
4.9/5  (40)
(40)
Suppose a boat engine leaks 938 milliliters of oil into a lake.The mass of this spilled oil is 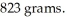 The oil will not mix with the lake water.Which statement is true?
The oil will not mix with the lake water.Which statement is true?
(Multiple Choice)
5.0/5  (42)
(42)
The wavelength of blue light is 0.00000045 m.Express this wavelength in scientific notation.
(Multiple Choice)
4.9/5  (35)
(35)
In multiplication and division calculations,the answer will have the same number of decimal places as the number carrying the fewest decimal places.
(True/False)
4.8/5  (36)
(36)
How many significant digits should be reported in the answer to the following calculation? (4.3 - 3.7)× 12.3 =
(Multiple Choice)
4.8/5  (28)
(28)
How many significant figures should be reported in the answer to the following calculation? (1.40)× (17.1)=
(Multiple Choice)
4.8/5  (34)
(34)
Trailing zeros at the end of a number,but before an implied decimal point,are ambiguous.
(True/False)
4.9/5  (36)
(36)
An object weighing 1.840 kg has a volume of 0.0015 m3.What is the density of the object in g/cm3?
(Multiple Choice)
4.9/5  (44)
(44)
What is the density of 96 mL of a liquid that has a mass of 90.5 g?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 61 - 80 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)