Exam 14: Acids and Bases
Exam 1: The Chemical World59 Questions
Exam 2: Measurement and Problem Solving131 Questions
Exam 3: Matter and Energy118 Questions
Exam 4: Atoms and Elements112 Questions
Exam 5: Molecules and Compounds110 Questions
Exam 6: Chemical Composition118 Questions
Exam 7: Chemical Reactions113 Questions
Exam 8: Quantities in Chemical Reactions115 Questions
Exam 9: Electrons in Atoms and the Periodic Table111 Questions
Exam 10: Chemical Bonding109 Questions
Exam 11: Gases123 Questions
Exam 12: Liquids, solids, and Intermolecular Forces115 Questions
Exam 13: Solutions122 Questions
Exam 14: Acids and Bases117 Questions
Exam 15: Chemical Equilibrium118 Questions
Exam 16: Oxidation and Reduction113 Questions
Exam 17: Radioactivity and Nuclear Chemistry115 Questions
Exam 18: Organic Chemistry109 Questions
Exam 19: Biochemistry110 Questions
Select questions type
Acid rain legislation targeted the release of which compound by industry?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following statements about a base are TRUE?
1.Bases are used in the manufacturing of soap.
2.Bases have a sour taste.
3.Fertilizer manufacture and cotton processing use bases.
(Multiple Choice)
4.7/5  (38)
(38)
What is the concentration of the hydronium ions in an acidic solution?
(Multiple Choice)
4.9/5  (33)
(33)
A neutralization reaction between KOH (aq)and H2SO4 (aq)would give which two products?
(Multiple Choice)
4.8/5  (35)
(35)
What is the pOH of a solution that has a OH- concentration equal to 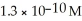 ?
?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements about acids are TRUE?
1.An acid is used in car batteries.
2.A component of vinegar is an acid.
3.Acids are used for cleaning metals.
(Multiple Choice)
4.8/5  (36)
(36)
Consider a 1.6 × 10-3 M solution of HNO3.Which of the following statements is NOT true?
(Multiple Choice)
4.9/5  (33)
(33)
What is the concentration of H+ in solution given the [OH⁻] = 2.54 × 10-4?
(Multiple Choice)
4.9/5  (35)
(35)
What is the pH of a solution that has a H⁺ concentration equal to 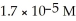 ?
?
(Multiple Choice)
4.8/5  (36)
(36)
Since diprotic acids such as H2CO3 have two ionizable protons,they will always behave as strong acids compared to monoprotic acids.
(True/False)
4.9/5  (37)
(37)
A simple buffer can be prepared by mixing significant amounts of a weak acid and its conjugate base.
(True/False)
4.8/5  (30)
(30)
What is the concentration of the hydronium ions in a neutral solution?
(Multiple Choice)
4.8/5  (32)
(32)
In the following reaction: NH4+ (aq)+ H2O (aq)→ NH3 (aq)+ H3O+ (aq)
(Multiple Choice)
4.7/5  (38)
(38)
Showing 21 - 40 of 117
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)