Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
The quantum numbers, n = 3, l = 1, ml = 0, ms = +1/2, represent an electron in a ____ subshell.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
Calculate the frequency of visible light having a wavelength of 486 nm.
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
C
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow. Of these colors, _______ has the longest wavelength.
Free
(Multiple Choice)
4.9/5  (48)
(48)
Correct Answer:
A
What is the maximum number of electrons in an atom that can have the following quantum numbers
N = 3 l = 1
(Multiple Choice)
4.9/5  (33)
(33)
Which ground-state atom has an electron configuration described by the following orbital diagram 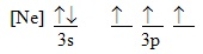
(Multiple Choice)
4.7/5  (37)
(37)
For all atoms of the same element, the 2s orbital is larger than the 1s orbital.
(True/False)
4.8/5  (25)
(25)
Using the figure below, categorize electromagnetic radiation with an energy of 6.6 x 10-16 J/photon. 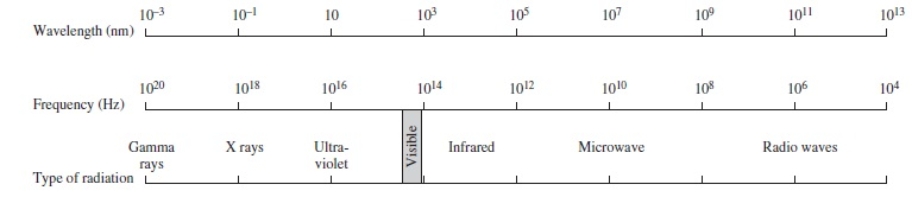
(Multiple Choice)
5.0/5  (34)
(34)
When the electron in a hydrogen atom falls from the n = 3 excited energy level to the ground state energy level, a photon with wavelength lis emitted. An electron having this same wavelength would have a velocity of
(Multiple Choice)
4.9/5  (47)
(47)
List the following sets of quantum numbers in order of increasing energy:
I. n = 4, l = 1, ml = 1, ms = +1/2
II. n = 3, l = 2, ml = -1, ms = +1/2
III. n = 4, l = 0, ml = 0, ms = +1/2
(Multiple Choice)
4.9/5  (43)
(43)
The ground-state electron configuration for an atom of indium is
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following, if any, is the correct ground state electron configuration for an iodine atom
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following scientists made this contribution to quantum: "It is impossible to know simultaneously both the momentum and the position of a particle with certainty."
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is diamagnetic both in its ground state and in all of its excited states
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following, if any, is the correct ground state electron configuration for a lead atom
(Multiple Choice)
4.8/5  (31)
(31)
Which element has the following ground-state electron configuration
[Kr]5s24d105p3
(Multiple Choice)
4.8/5  (29)
(29)
A single pulse of a laser yields an average of 5.00 * 1018 photons with l= 633 nm. If melting ice to water at 0 C requires 6.01 kJ/mol, what is the fewest number of laser pulses needed to melt 10.0 g of ice
(Multiple Choice)
4.8/5  (32)
(32)
How many unpaired electrons does an atom of sulfur have in its ground state
(Multiple Choice)
4.7/5  (43)
(43)
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow. Of these colors, _______ has the most energy.
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)