Exam 7: Quantum Theory and the Electronic Structure of Atoms
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
If a hydrogen atom and a helium atom are traveling at the same speed,
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following, if any, is the correct ground state electron configuration for Cr
(Multiple Choice)
4.9/5  (31)
(31)
A ground-state atom of iron has ___ unpaired electrons and is _____.
(Multiple Choice)
4.9/5  (41)
(41)
When photons with a wavelength of 310. nm strike a magnesium plate, the maximum velocity of the ejected electrons is 3.45 * 105 m/s. Calculate the binding energy of electrons to the magnesium surface.
(Multiple Choice)
4.8/5  (44)
(44)
The electron in a hydrogen atom falls from an excited energy level to the ground state in two steps, causing the emission of photons with wavelengths of 1870 nm and 102.5 nm, respectively. What is the principal quantum number or shell of the initial excited energy level from which the electron falls
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following wavelengths of electromagnetic radiation has the highest energy
(Multiple Choice)
4.8/5  (43)
(43)
Which one of the following sets of quantum numbers is not possible \nobreakspace\nobreakspace\nobreakspace\nobreakspace\nobreakspace n l A. 4 3 -2 +1/2 B. 3 0 1 -1/2 C. 3 0 0 +1/2 D. 2 1 1 -1/2 E. 2 0 0 +1/2
(Short Answer)
4.7/5  (36)
(36)
Calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. Recall that for hydrogen En = -2.18 * 10-18 J(1/n2).
(Multiple Choice)
4.8/5  (33)
(33)
If we take away two electrons from the outer shell of calcium, it would have the same electron configuration as what element
(Multiple Choice)
4.9/5  (38)
(38)
Using the figure below, categorize electromagnetic radiation with an energy of 6.7 x 10-18 J/photon. 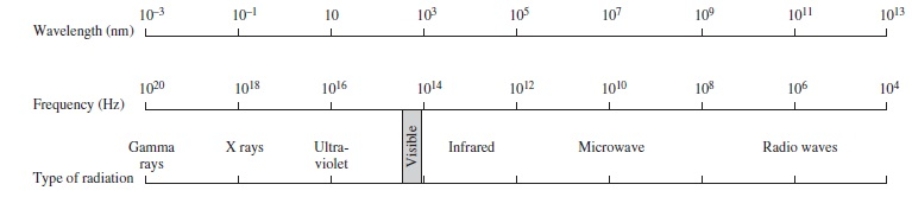
(Multiple Choice)
4.8/5  (35)
(35)
The frequency of the emitted light from a cesium atom is an intensive property.
(True/False)
4.8/5  (39)
(39)
Calculate the frequency of visible light having a wavelength of 686 nm.
(Multiple Choice)
4.9/5  (44)
(44)
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 6 to the n = 3 principal energy level. Recall that for hydrogen En = -2.18 * 10-18 J(1/n2).
(Multiple Choice)
4.8/5  (45)
(45)
The quantum numbers, n = 4, l = 3, ml = 2, ms = +1/2, represent an electron in a ___ subshell
(Multiple Choice)
4.9/5  (45)
(45)
Which element has the following ground-state electron configuration
[Kr]5s14d5
(Multiple Choice)
4.8/5  (45)
(45)
The colors of the visible spectrum are blue, green, orange, red, violet, and yellow. Of these colors, ______ has the shortest wavelength.
(Multiple Choice)
4.8/5  (32)
(32)
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm
(Multiple Choice)
4.8/5  (35)
(35)
What is the maximum number of electrons in an atom that can have the following set of quantum numbers
N = 3 l = 2 ml = -2
(Multiple Choice)
4.9/5  (34)
(34)
Showing 61 - 80 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)