Exam 11: Intermolecular Forces and Liquids and Solids
Exam 1: Chemistry: The Study of Change175 Questions
Exam 2: Atoms Molecules and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions192 Questions
Exam 5: Gases130 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts136 Questions
Exam 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic146 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics131 Questions
Exam 14: Chemical Equilibrium119 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria145 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry128 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metals Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry66 Questions
Exam 25: Synthetic and Natural Organic Polymers46 Questions
Select questions type
The intermolecular forces present in CH3NH2 include which of the following
I. dipole-dipole
II. ion-dipole
III. dispersion
IV. hydrogen bonding
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
Indicate all the types of intermolecular forces of attraction in CHCl3(l).
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
Which type of intermolecular force is the strongest (ionic, ion-dipole, dipole-dipole, hydrogen bonding, dispersion)
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
E
Which two properties are more typical of molecular compounds than of ionic compounds
1. They are gases or liquids at room temperature.
2. They have high melting points.
3. Solids do not conduct electricity, but liquids do.
4. Atoms share electrons.
(Multiple Choice)
4.9/5  (29)
(29)
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure. The edge length of this cubic unit cell is 540.9 pm. Determine the density of zincblende.
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following properties is not influenced by hydrogen bonding
(Multiple Choice)
4.8/5  (30)
(30)
Polyethylene plastic consists of long chains of carbon atoms, each of which is also bonded to hydrogens as shown below: 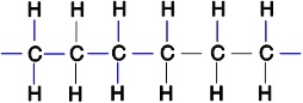 Water forms beads when placed on a polyethylene surface. Why
Water forms beads when placed on a polyethylene surface. Why
(Multiple Choice)
4.8/5  (33)
(33)
Arrange the following in order of increasing melting point: NaCl, H2O, CH4, C6H4(OH)2.
(Multiple Choice)
4.9/5  (43)
(43)
Indicate all the types of intermolecular forces of attraction in He(l).
(Multiple Choice)
4.9/5  (37)
(37)
The molar enthalpy of vaporization of hexane (C6H14) is 28.9 kJ/mol, and its normal boiling point is 68.73 C. What is the vapor pressure of hexane at 25 C
(Multiple Choice)
4.9/5  (32)
(32)
Given that the heat of vaporization of mercury is 59.0 kJ/mol and the vapor pressure of mercury is 0.0017 torr at 25 C, calculate the normal boiling point of mercury.
(Multiple Choice)
4.9/5  (37)
(37)
Which one of the following substances should exhibit hydrogen bonding in the liquid state
(Multiple Choice)
4.9/5  (40)
(40)
The atomic planes in a graphite crystal are separated by 335 pm. At what angle would you find the first-order (n = 1) diffraction of 0.154 nm X-rays from a graphite crystal
(Multiple Choice)
4.8/5  (24)
(24)
How much enthalpy is necessary to heat 10.0 g of solid benzene (C6H6) at 0.0 C to benzene vapor at 100 C
melting point 5. boiling point 80. specific heat of solid benzene 1.52/\cdot specific heat of liquid benzene 1.73/\cdot specific heat of benzene vapor 1.06/\cdot \Delta 9.9/ \Delta 30.8/
(Multiple Choice)
4.9/5  (30)
(30)
Crystals of elemental sulfur are easily crushed, and melt at 113 C. Liquid sulfur does not conduct electricity. What kind of crystal is this
(Multiple Choice)
4.7/5  (39)
(39)
The intermolecular forces present in HSCH2CH2SH include which of the following
I. dipole-dipole
II. ion-dipole
III. dispersion
IV. hydrogen bonding
(Multiple Choice)
4.8/5  (47)
(47)
Magnesium oxide, MgO, melts at 2,800 C and is very hard. The liquid conducts electricity very well. What kind of crystal is this
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the following is an example of a covalent network solid
(Multiple Choice)
4.9/5  (28)
(28)
Each of the following substances is a gas at 25 C and 1 atmosphere pressure. Which one will liquefy most easily when compressed at a constant temperature
(Multiple Choice)
4.7/5  (43)
(43)
Potassium bromide, KBr, crystallizes like NaCl in a face-centered lattice. The ionic radii of K+ and Br- ions are 133 pm and 195 pm, respectively. Assuming that all Br- ions are positioned in the face and corners of the unit cell, while the K+ ions are positioned along the edge alternating between anions, calculate the length of a unit cell edge.
(Multiple Choice)
4.9/5  (42)
(42)
Showing 1 - 20 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)