Exam 8: Atoms and Periodic Properties
Exam 1: What Is Science30 Questions
Exam 2: Motion30 Questions
Exam 3: Energy30 Questions
Exam 4: Heat and Temperature30 Questions
Exam 5: Wave Motions and Sound30 Questions
Exam 6: Electricity30 Questions
Exam 7: Light35 Questions
Exam 8: Atoms and Periodic Properties30 Questions
Exam 9: Chemical Bonds30 Questions
Exam 10: Chemical Reactions30 Questions
Exam 11: Water and Solutions30 Questions
Exam 12: Organic Chemistry30 Questions
Exam 13: Nuclear Reactions30 Questions
Exam 14: The Universe30 Questions
Exam 15: The Solar System30 Questions
Exam 16: Earth in Space30 Questions
Exam 17: Rocks and Minerals30 Questions
Exam 18: Plate Tectonics30 Questions
Exam 19: Building Earths Surface30 Questions
Exam 20: Shaping Earths Surface30 Questions
Exam 21: Geologic Time30 Questions
Exam 22: The Atmosphere of Earth29 Questions
Exam 23: Weather and Climate30 Questions
Exam 24: Earths Waters30 Questions
Select questions type
John Dalton reasoned that atoms exist from the evidence that
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
Photons of which of the following colors of light possess the greatest amount of energy?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
The atomic number of an element is the number of
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
A
The atomic number of an element is the total number of protons and neutrons in the nucleus.
(True/False)
4.9/5  (30)
(30)
According to the quantum mechanical model, electrons exist in
(Multiple Choice)
4.7/5  (25)
(25)
Heisenberg's uncertainty principle says that you cannot know the momentum or the position of an electron exactly.
(True/False)
4.8/5  (29)
(29)
Millikan found that all of the oil droplets in his apparatus carried a charge that was an integer multiple of one particular value.
(True/False)
4.9/5  (38)
(38)
In the Bohr model of the atom, the energy state of an electron could be described with one number.The quantum mechanical model of the atom requires how many numbers to do the same?
(Multiple Choice)
4.9/5  (35)
(35)
The proposal that matter, like light, exhibits wave-like behavior was
(Multiple Choice)
4.9/5  (43)
(43)
Identify the number of protons, neutrons, and electrons in an atom of
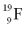 .
.
(Multiple Choice)
4.7/5  (36)
(36)
The fact that wavelengths of the four lines in the Balmer series fit a regular pattern was evidence supporting the idea that
(Multiple Choice)
4.8/5  (36)
(36)
When Rutherford found that some of the alpha particles fired at the gold foil were widely deflected, he concluded that
(Multiple Choice)
4.7/5  (32)
(32)
Einstein proposed that electrons on the surface of a metal gradually absorb energy from photons until they have enough energy to leave the surface.
(True/False)
4.9/5  (36)
(36)
J.J.Thomson reasoned that cathode rays were really charged particles because
(Multiple Choice)
4.9/5  (24)
(24)
Protons are so much more massive than electrons that you can neglect the mass of electrons when determining the mass of an atom.
(True/False)
4.9/5  (29)
(29)
The quantum mechanical model of the atom differs from the Bohr model in that it
(Multiple Choice)
4.9/5  (32)
(32)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)