Exam 11: Water and Solutions
Exam 1: What Is Science30 Questions
Exam 2: Motion30 Questions
Exam 3: Energy30 Questions
Exam 4: Heat and Temperature30 Questions
Exam 5: Wave Motions and Sound30 Questions
Exam 6: Electricity30 Questions
Exam 7: Light35 Questions
Exam 8: Atoms and Periodic Properties30 Questions
Exam 9: Chemical Bonds30 Questions
Exam 10: Chemical Reactions30 Questions
Exam 11: Water and Solutions30 Questions
Exam 12: Organic Chemistry30 Questions
Exam 13: Nuclear Reactions30 Questions
Exam 14: The Universe30 Questions
Exam 15: The Solar System30 Questions
Exam 16: Earth in Space30 Questions
Exam 17: Rocks and Minerals30 Questions
Exam 18: Plate Tectonics30 Questions
Exam 19: Building Earths Surface30 Questions
Exam 20: Shaping Earths Surface30 Questions
Exam 21: Geologic Time30 Questions
Exam 22: The Atmosphere of Earth29 Questions
Exam 23: Weather and Climate30 Questions
Exam 24: Earths Waters30 Questions
Select questions type
Which of the following are properties of basic solutions?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
The presence of solute molecules makes a solution freeze at a lower temperature than the pure solvent.
Free
(True/False)
4.9/5  (38)
(38)
Correct Answer:
True
Water solutions of ionic substances that conduct electricity are called
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following solutions is likely to have a pH less than 7?
(Multiple Choice)
4.8/5  (32)
(32)
Water containing large amounts of Mg2+ and Ca2+ions is said to be hard because it is hard to make soap lather in the water.
(True/False)
4.9/5  (34)
(34)
The water hardness in an area is reported as 700 ppm total dissolved solids.This is the same concentration as
(Multiple Choice)
4.9/5  (39)
(39)
A solution is saturated when no more of a solute will dissolve in a solvent.
(True/False)
4.8/5  (27)
(27)
What do solutions of acids, bases, and salts have in common? They
(Multiple Choice)
4.8/5  (33)
(33)
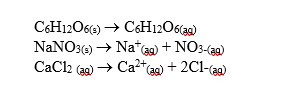 -Aqueous solutions with one mole of each solid in equal volumes of water are prepared.Which solution would have the lowest freezing point?
-Aqueous solutions with one mole of each solid in equal volumes of water are prepared.Which solution would have the lowest freezing point?
(Multiple Choice)
4.7/5  (26)
(26)
Cooks add a pinch of salt to water to increase its boiling point since it is known that solutions boil at a higher temperature than pure water.
(True/False)
4.8/5  (39)
(39)
Air is considered to be a homogeneous mixture that is 79 percent nitrogen gas, 20 percent oxygen gas, and 1 percent all the other gases.In this mixture, nitrogen can be considered
(Multiple Choice)
4.8/5  (31)
(31)
The solubility of a gas in water decreases as the water temperature increases.
(True/False)
4.9/5  (31)
(31)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)