Exam 11: Introduction to Organic Chemistry
Exam 1: Introduction153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Stoichiometry168 Questions
Exam 4: Reactions in Aqueous Solution156 Questions
Exam 5: Gases109 Questions
Exam 6: Energy Relationships in Chemical Reactions105 Questions
Exam 7: The Electronic Structure of Atoms115 Questions
Exam 8: The Periodic Table119 Questions
Exam 9: Chemical Bonding I: the Covalent Bond118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals120 Questions
Exam 11: Introduction to Organic Chemistry57 Questions
Exam 12: Intermolecular Forces and Liquids and Solids138 Questions
Exam 13: Physical Properties of Solutions109 Questions
Exam 14: Chemical Kinetics114 Questions
Exam 15: Chemical Equilibrium99 Questions
Exam 16: Acids and Bases163 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria92 Questions
Exam 18: Thermodynamics112 Questions
Exam 19: Redox Reactions and Electrochemistry138 Questions
Exam 20: The Chemistry of Coordination Compounds76 Questions
Exam 21: Nuclear Chemistry112 Questions
Exam 22: Organic Polymerssynthetic and Natural42 Questions
Select questions type
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 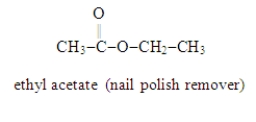
Free
(Essay)
4.7/5  (47)
(47)
Correct Answer:
CH3-COOH and CH3-CH2-OH
Which of these pairs are geometric isomers?
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
D
Which one of these choices is the formula for an aldehyde?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
C
Which one of these hydrocarbon chains would have the highest octane rating?
(Multiple Choice)
4.8/5  (36)
(36)
A particular structural isomer of C6H14 is shown below. 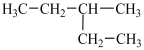 Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
(Multiple Choice)
4.9/5  (44)
(44)
The systematic name for the compound with the following structural formula is 4,5-dimethyl-2-hexene. 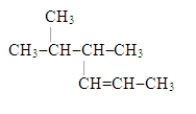
(True/False)
4.9/5  (45)
(45)
The two molecules represented below are examples of CH3-CH2-O-CH2CH3 CH3CH2CH2CH2-OH
(Multiple Choice)
4.9/5  (45)
(45)
The name for the compound with the formula CH3CH2CH2CH2OH is
(Multiple Choice)
4.7/5  (39)
(39)
Which of these is the systematic name for the compound represented below? 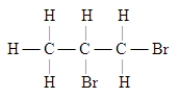
(Multiple Choice)
4.7/5  (38)
(38)
Which is the product of the reaction of one mole of HCl with one mole of 1-butyne?
(Multiple Choice)
4.9/5  (35)
(35)
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 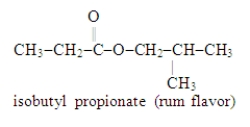
(Essay)
4.8/5  (40)
(40)
Which choice gives the structures of the reaction products when the ester below is hydrolyzed in acid solution? 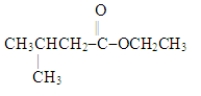
(Multiple Choice)
4.7/5  (36)
(36)
Showing 1 - 20 of 57
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)