Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
A sp2 hybridized terminal oxygen atom (bonded to one other atom only)with 2 lone pairs of electrons has what type of bonding?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
In which of the following would the bonding be weakened with the subtraction of an electron to form a positive molecular ion?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
Consider the species N2-, N2, and N2+.Which of these species will be paramagnetic?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
C
A bonding molecular orbital is of lower energy (more stable)than the atomic orbitals from which it was formed.
(True/False)
4.8/5  (32)
(32)
According to Valence Bond Theory which orbital is left vacant in the molecule BF3?
(Multiple Choice)
4.7/5  (29)
(29)
A central atom with 5 electron pairs (single bonds and/or lone pairs of electrons)could have which of the following molecular geometries? I.Trigonal bipyramidal
II.Seesaw
III.T-shaped
IV.Linear
(Multiple Choice)
4.8/5  (40)
(40)
A sp2 hybridized central atom has what angles between its hybrid orbitals?
(Multiple Choice)
4.8/5  (38)
(38)
Predict the molecular geometry and polarity of the SO2 molecule.
(Multiple Choice)
4.8/5  (33)
(33)
A sp3d hybridized central atom has what angles between its hybrid orbitals?
(Multiple Choice)
4.8/5  (50)
(50)
An sp2 hybridized central carbon atom with no lone pairs of electrons has what type of bonding?
(Multiple Choice)
4.7/5  (36)
(36)
Give the number of lone pairs around the central atom and the molecular geometry of IF5.
(Multiple Choice)
4.8/5  (26)
(26)
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever.What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 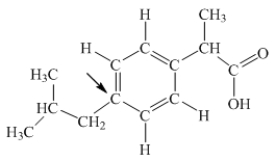
(Multiple Choice)
4.8/5  (39)
(39)
In benzene (C6H6), what is the hybridization of each carbon atom?
(Short Answer)
4.8/5  (38)
(38)
Indicate the type of hybrid orbitals used by the central atom in CCl4.
(Multiple Choice)
4.8/5  (26)
(26)
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents.What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below? 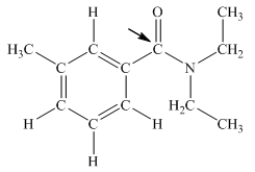
(Multiple Choice)
4.7/5  (35)
(35)
Consider the species Cl2+, Cl2, and Cl2-.Which of these species will be paramagnetic?
(Multiple Choice)
4.7/5  (31)
(31)
Showing 1 - 20 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)