Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
Exam 1: Chemistry: the Study of Change168 Questions
Exam 2: Atoms, Molecules, and Ions156 Questions
Exam 3: Mass Relationships in Chemical Reactions194 Questions
Exam 4: Reactions in Aqueous Solutions186 Questions
Exam 5: Gases121 Questions
Exam 6: Thermochemistry118 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms136 Questions
Exam 8: Periodic Relationships Among the Elements144 Questions
Exam 9: Chemical Bonding I: Basic Concepts137 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals147 Questions
Exam 11: Intermolecular Forces and Liquids and Solids149 Questions
Exam 12: Physical Properties of Solutions122 Questions
Exam 13: Chemical Kinetics130 Questions
Exam 14: Chemical Equilibrium109 Questions
Exam 15: Acids and Bases178 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria131 Questions
Exam 17: Entropy Free Energy and Equilibrium128 Questions
Exam 18: Electrochemistry154 Questions
Exam 19: Nuclear Chemistry133 Questions
Exam 20: Chemistry in the Atmosphere50 Questions
Exam 21: Metallurgy and the Chemistry of Metals63 Questions
Exam 22: Nonmetallic Elements and Their Compounds52 Questions
Exam 23: Transition Metal Chemistry and Coordination Compounds92 Questions
Exam 24: Organic Chemistry67 Questions
Exam 25: Synthetic and Natural Organic Polymers50 Questions
Select questions type
According to VSEPR theory, which of the following triatomic ions should be linear: N3-, I3-, NO2-, ClO2-, SCN-.
(Essay)
4.9/5  (42)
(42)
Give the number of lone pairs around the central atom and the molecular geometry of SeF4.
(Multiple Choice)
4.8/5  (42)
(42)
Give the number of lone pairs around the central atom and the geometry of the ion NO2-.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following species has the largest dipole moment (i.e., is the most polar)?
(Multiple Choice)
4.9/5  (40)
(40)
Ozone (O3)is an allotropic form of oxygen.Use VSEPR theory to predict the shape of the ozone molecule.
(Short Answer)
5.0/5  (28)
(28)
A molecule with polar bonds must be a polar molecule in all cases.
(True/False)
4.9/5  (30)
(30)
Which of the following molecules is polar?
A.CH3OH
B.H2O
C.CH3OCH3
(Short Answer)
4.8/5  (37)
(37)
The geometry of the hybrid orbitals about a central atom with sp3d hybridization is:
(Multiple Choice)
4.9/5  (35)
(35)
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents.What is the hybridization state of the nitrogen atom in the structure of DEET shown below? 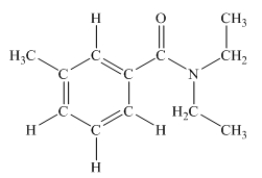
(Multiple Choice)
4.8/5  (38)
(38)
List all of the bond angles present in a molecule with a trigonal bipyramidal geometry.
(Short Answer)
4.8/5  (43)
(43)
Which of the following molecules has polar bonds but is a nonpolar molecule? BF3, CCl4, CO, SF4
(Short Answer)
5.0/5  (38)
(38)
Using periodic trends, arrange the following molecules in order of increasing dipole moment: NH3, PH3, AsH3.
(Essay)
4.9/5  (39)
(39)
According to the VSEPR theory, the actual F -As -F bond angles in the AsF4- ion are predicted to be
(Multiple Choice)
4.8/5  (35)
(35)
According to the VSEPR theory, all of the electron pair-electron pair repulsions about the central atom in PCl3 are of equal magnitude.
(True/False)
4.8/5  (40)
(40)
According to the VSEPR theory, the geometrical structure of PF5 is
(Short Answer)
4.8/5  (41)
(41)
According to the VSEPR theory, the molecular geometry of boron trichloride is
(Multiple Choice)
4.9/5  (33)
(33)
Showing 101 - 120 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)