Exam 10: Acids and Bases
Exam 1: Matter and Measurements143 Questions
Exam 2: Atoms and the Periodic Table91 Questions
Exam 3: Ionic Compounds75 Questions
Exam 4: Molecular Compounds75 Questions
Exam 5: Classification and Balancing of Chemical Reactions53 Questions
Exam 6: Chemical Reactions: Mole and Mass Relationships46 Questions
Exam 7: Chemical Reactions: Energy, rates, and Equilibrium73 Questions
Exam 8: Gases, liquids, and Solids73 Questions
Exam 9: Solutions110 Questions
Exam 10: Acids and Bases102 Questions
Exam 11: Nuclear Chemistry88 Questions
Exam 12: Introduction to Organic Chemistry: Alkanes83 Questions
Exam 13: Alkenes, alkynes, and Aromatic Compounds85 Questions
Exam 14: Some Compounds With Oxygen, sulfur, or a Halogen94 Questions
Exam 15: Amines46 Questions
Exam 16: Aldehydes and Ketones77 Questions
Exam 17: Carboxylic Acids and Their Derivatives84 Questions
Exam 18: Amino Acids and Proteins78 Questions
Exam 19: Enzymes and Vitamins75 Questions
Exam 20: The Generation of Biochemical Energy66 Questions
Exam 21: Carbohydrates72 Questions
Exam 22: Carbohydrate Metabolism60 Questions
Exam 23: Lipids79 Questions
Exam 24: Lipid Metabolism58 Questions
Exam 25: Nucleic Acids and Protein Synthesis67 Questions
Exam 26: Genomics38 Questions
Exam 27: Protein and Amino Acid Metabolism47 Questions
Exam 28: Chemical Messengers: Hormones, neurotransmitters, and Drugs73 Questions
Exam 29: Body Fluids63 Questions
Select questions type
Which compound is manufactured in larger quantities in the U.S.than any other industrial chemical?
(Multiple Choice)
4.8/5  (34)
(34)
The normality of a solution prepared by dissolving 50.0 g of Ca(OH)2 in water to make 250.mL solution is ________ N.
(Multiple Choice)
4.8/5  (36)
(36)
Consider the following reaction: NO2- + HCO3- 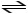 CO3-2 + HNO2
Identify the acid,base,conjugate acid and conjugate base.
CO3-2 + HNO2
Identify the acid,base,conjugate acid and conjugate base.
(Essay)
4.7/5  (37)
(37)
All of the following species are involved in the blood buffer system except ________.
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following pH's corresponds to a weakly acidic solution?
(Multiple Choice)
4.7/5  (31)
(31)
What is the normality of a solution containing 49 g of H2SO4 in enough water to make 400 mL of solution?
(Multiple Choice)
4.8/5  (39)
(39)
Which compound has a very large value of Ka in aqueous solution?
(Multiple Choice)
4.8/5  (34)
(34)
Which compound produces an acidic solution when dissolved in water?
(Multiple Choice)
4.9/5  (34)
(34)
Which net ionic equation correctly represents the neutralization of a solution of barium hydroxide by a solution of nitric acid?
(Multiple Choice)
4.9/5  (42)
(42)
How many mL of 0.150 M NaOH are needed to neutralize 50.00 mL of a 0.120 M solution of H2SO4?
(Multiple Choice)
4.7/5  (38)
(38)
To prepare a buffer using sodium phosphate,which of the following would also be needed?
(Multiple Choice)
4.7/5  (34)
(34)
What is the pH of a solution in which the hydrogen ion concentration is 5.1 × 10-8 M?
(Multiple Choice)
4.7/5  (40)
(40)
What is the concentration of a nitric acid solution if a 10.00 mL sample of the acid requires 31.25 mL of 0.135 M KOH for neutralization?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following pH's corresponds to a neutral solution?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 21 - 40 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)