Exam 10: Acids and Bases
Exam 1: Matter and Measurements143 Questions
Exam 2: Atoms and the Periodic Table91 Questions
Exam 3: Ionic Compounds75 Questions
Exam 4: Molecular Compounds75 Questions
Exam 5: Classification and Balancing of Chemical Reactions53 Questions
Exam 6: Chemical Reactions: Mole and Mass Relationships46 Questions
Exam 7: Chemical Reactions: Energy, rates, and Equilibrium73 Questions
Exam 8: Gases, liquids, and Solids73 Questions
Exam 9: Solutions110 Questions
Exam 10: Acids and Bases102 Questions
Exam 11: Nuclear Chemistry88 Questions
Exam 12: Introduction to Organic Chemistry: Alkanes83 Questions
Exam 13: Alkenes, alkynes, and Aromatic Compounds85 Questions
Exam 14: Some Compounds With Oxygen, sulfur, or a Halogen94 Questions
Exam 15: Amines46 Questions
Exam 16: Aldehydes and Ketones77 Questions
Exam 17: Carboxylic Acids and Their Derivatives84 Questions
Exam 18: Amino Acids and Proteins78 Questions
Exam 19: Enzymes and Vitamins75 Questions
Exam 20: The Generation of Biochemical Energy66 Questions
Exam 21: Carbohydrates72 Questions
Exam 22: Carbohydrate Metabolism60 Questions
Exam 23: Lipids79 Questions
Exam 24: Lipid Metabolism58 Questions
Exam 25: Nucleic Acids and Protein Synthesis67 Questions
Exam 26: Genomics38 Questions
Exam 27: Protein and Amino Acid Metabolism47 Questions
Exam 28: Chemical Messengers: Hormones, neurotransmitters, and Drugs73 Questions
Exam 29: Body Fluids63 Questions
Select questions type
Consider the reaction: H2SO3 + HCO3- 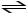 H2CO3 + HSO3-
a)Identify the acid,base,conjugate acid and conjugate base
b)Identify two substances from the reaction which could be used to prepare a buffer.
H2CO3 + HSO3-
a)Identify the acid,base,conjugate acid and conjugate base
b)Identify two substances from the reaction which could be used to prepare a buffer.
(Essay)
4.8/5  (38)
(38)
If the concentration of H3O+ is 3.5 × 10-3 M,the concentration of OH- is ________ M.
(Multiple Choice)
4.9/5  (46)
(46)
According to Brønsted-Lowry theory,acid-base reactions can be described as ________ reactions.
(Multiple Choice)
4.9/5  (36)
(36)
Which statement concerning Arrhenius acid-base theory is not correct?
(Multiple Choice)
4.8/5  (33)
(33)
Explain the term "amphoteric." Use the hydrogen carbonate ion,HCO3- to illustrate amphoteric behavior.
(Essay)
4.8/5  (34)
(34)
What is the normality of a solution containing 100.g HNO3 in 500.mL of solution?
(Multiple Choice)
4.7/5  (40)
(40)
The classification of an acid or a base as weak or strong is determined by
(Multiple Choice)
4.9/5  (32)
(32)
All of the statements regarding equivalents of acids and bases are true except
(Multiple Choice)
4.9/5  (37)
(37)
Which equation correctly represents the neutralization of aluminum hydroxide by sulfuric acid?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 81 - 100 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)