Exam 12: The Gaseous State of Matter
Exam 1: An Introduction to Chemistry56 Questions
Exam 2: Standards for Measurement106 Questions
Exam 3: Elements and Compounds108 Questions
Exam 4: Properties of Matter112 Questions
Exam 5: Early Atomic Theory and Structure112 Questions
Exam 6: Nomenclature of Inorganic Compounds113 Questions
Exam 7: Quantitative Composition of Compounds111 Questions
Exam 8: Chemical Equations113 Questions
Exam 9: Calculations From Chemical Equations101 Questions
Exam 10: Modern Atomic Theory and the Periodic Table99 Questions
Exam 11: Chemical Bonds: the Formation of Compounds From Atoms102 Questions
Exam 12: The Gaseous State of Matter100 Questions
Exam 13: Liquids100 Questions
Exam 14: Solutions100 Questions
Exam 15: Acids, Bases, and Salts100 Questions
Exam 16: Chemical Equilibrium101 Questions
Exam 17: Oxidationreduction100 Questions
Exam 18: Nuclear Chemistry102 Questions
Exam 19: Introduction to Organic Chemistry Online Only99 Questions
Exam 20: Introduction to Biochemistry Online Only105 Questions
Select questions type
Look at the apparatus shown below.Inside each chamber there is a sample of a gas at the specified volume and pressure.Once the stopcock is opened,what will be the total pressure inside the system.The whole process is done under constant temperature. 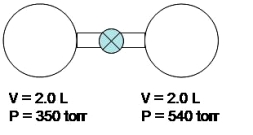
(Multiple Choice)
4.8/5  (43)
(43)
What volume of carbon dioxide gas is produced when 3.00 L of oxygen gas react with 7.5 L of carbon monoxide in the following equation?
2 CO(g)+ O2(g) 2 CO2(g)
(Multiple Choice)
4.9/5  (36)
(36)
A gas mixture containing N2 and O2 was kept inside a 2.00 L container at a temperature of 23.0°C and a total pressure of 1.00 atm.The partial pressure of oxygen was 0.722 atm.How many grams of nitrogen were present in the gas mixture?
(Multiple Choice)
4.8/5  (33)
(33)
A sample of gas has a volume of 3.40 L at 10.0 °C.What will be its volume at 100.0°C, pressure remaining constant?
(Multiple Choice)
4.9/5  (32)
(32)
An 11.2 L sample of nitrogen contains the same number of molecules as
(Multiple Choice)
4.8/5  (37)
(37)
A 400.mL sample of hydrogen gas is collected over water at 20.0 ° C and 760.0 torr.
The vapor pressure of water at 20.0°C is 17.5 torr.What volume will the dry hydrogen gasoccupy at 20.0 ° C and 760.torr?
(Short Answer)
4.9/5  (39)
(39)
What volume of sulfur dioxide gas will be consumed when 12.0 L of oxygen is consumed in the following equation? 2 SO2(g)+ O2(g) 2 SO3(g)
(Multiple Choice)
4.8/5  (40)
(40)
A sample of gas has a volume of 850.mL at 23.0 ° C and 1.10 atm.The temperature is increased to 33.0 ° C,at what pressure will its volume be 900.mL?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following gases would have the greatest kinetic energy at 300 K?
(Multiple Choice)
4.8/5  (40)
(40)
A 3.00 L sample of a gas at a pressure of 4.00 atm is compressed to 2.00 L at a constant temperature.What is the pressure of the gas?
(Multiple Choice)
4.8/5  (29)
(29)
What volume of dinitrogen pentoxide gas is produced when 500.mL of oxygen react completely in the following equation? 2 N2(g)+ 5 O2(g) 2 N2O5(g)
(Multiple Choice)
4.7/5  (37)
(37)
A 64.0 g sample of oxygen contains the same number of molecules as
(Multiple Choice)
4.7/5  (38)
(38)
What mass of helium would be contained within a 1.8 L balloon at a pressure of 766 torr and a temperature of 24.5°C?
(Multiple Choice)
4.8/5  (44)
(44)
At STP,3.00 L of nitrogen gas contains the same number of molecules as
(Multiple Choice)
4.9/5  (35)
(35)
What is the volume of 1.40 moles of nitrogen gas at 20.0 ° C and 770.torr?
(Multiple Choice)
4.9/5  (40)
(40)
What is the molar mass of a gas if 18.0 L of the gas at STP has a mass of 41.6 grams?
(Multiple Choice)
4.8/5  (28)
(28)
A sample of gas is in a cylinder of fixed volume.As the temperature of the sampleincreases,its internal pressure will
(Multiple Choice)
4.9/5  (34)
(34)
Showing 21 - 40 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)