Exam 12: The Gaseous State of Matter
Exam 1: An Introduction to Chemistry56 Questions
Exam 2: Standards for Measurement106 Questions
Exam 3: Elements and Compounds108 Questions
Exam 4: Properties of Matter112 Questions
Exam 5: Early Atomic Theory and Structure112 Questions
Exam 6: Nomenclature of Inorganic Compounds113 Questions
Exam 7: Quantitative Composition of Compounds111 Questions
Exam 8: Chemical Equations113 Questions
Exam 9: Calculations From Chemical Equations101 Questions
Exam 10: Modern Atomic Theory and the Periodic Table99 Questions
Exam 11: Chemical Bonds: the Formation of Compounds From Atoms102 Questions
Exam 12: The Gaseous State of Matter100 Questions
Exam 13: Liquids100 Questions
Exam 14: Solutions100 Questions
Exam 15: Acids, Bases, and Salts100 Questions
Exam 16: Chemical Equilibrium101 Questions
Exam 17: Oxidationreduction100 Questions
Exam 18: Nuclear Chemistry102 Questions
Exam 19: Introduction to Organic Chemistry Online Only99 Questions
Exam 20: Introduction to Biochemistry Online Only105 Questions
Select questions type
What volume of oxygen is consumed when 3.00 moles of carbon monoxide is produced in the following equation? 2 C(s)+ O2(g) 2 CO(g)
(Multiple Choice)
4.8/5  (40)
(40)
Which gas has approximately the same density as carbon dioxide gas at STP?
(Multiple Choice)
4.9/5  (41)
(41)
A sample of oxygen is collected over water at 22 ° C and 762 torr.What is the partial pressure of the dry oxygen? The vapor pressure of water at 22°C is 19.8 torr.
(Multiple Choice)
4.8/5  (37)
(37)
A sample of gas is in a cylinder of fixed volume.At 30.0 °C its internal pressure is 3200. torr.What will be its pressure at 12.0 ° C?
(Multiple Choice)
4.9/5  (38)
(38)
What volume of chlorine is consumed when 12.0 g of HCl is produced in the following equation? H2(g)+ Cl2(g) 2 HCl(g)
(Multiple Choice)
4.8/5  (27)
(27)
The pressure of a gas is directly proportional to the number of gas molecules present.
(True/False)
4.8/5  (37)
(37)
A sample of a gas is held at constant temperature.If the number of moles of gas in the sample is doubled while the pressure is halved,what will happen to the volume of the gas sample?
(Multiple Choice)
4.9/5  (35)
(35)
A small,0.500 mL,bubble forms at the bottom of a lake where the temperature is 2.0 °C and the pressure is 2.40 atm.What volume will the bubble occupy near the surface wherethe temperature is 32.0 ° C and the pressure is 1.10 atm?
(Short Answer)
4.8/5  (31)
(31)
How many moles are present in 10.0 L of nitrogen gas at STP?
(Multiple Choice)
4.8/5  (39)
(39)
What mass of water is produced when 10.0 L of oxygen react completely in the following equation? 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)
(Multiple Choice)
4.9/5  (39)
(39)
The density of an unknown gas at STP is 1.63 g/L.Is the gas H2(g),N2(g),HCl(g),or CO2(g)?
(Short Answer)
4.9/5  (46)
(46)
What is the molar mass of a gas if 30.0 L of the gas at STP has a mass of 16.0 grams?
(Multiple Choice)
4.8/5  (35)
(35)
The following figure represents one mole of an ideal gas in a container fit with a movable piston. 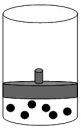 Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
(Multiple Choice)
4.7/5  (35)
(35)
A 250.mL sample of methane gas is collected over water at 21.0 ° C and 768.0 torr.
The vapor pressure of water at 21.0 ° C is 18.7 torr.What volume will the dry methane gasoccupy at STP?
(Short Answer)
4.9/5  (33)
(33)
The following figure shows 1 mol of an ideal gas inside a container with a movable piston.  If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.
If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)