Exam 15: Acids, Bases, and Salts
Exam 1: An Introduction to Chemistry56 Questions
Exam 2: Standards for Measurement106 Questions
Exam 3: Elements and Compounds108 Questions
Exam 4: Properties of Matter112 Questions
Exam 5: Early Atomic Theory and Structure112 Questions
Exam 6: Nomenclature of Inorganic Compounds113 Questions
Exam 7: Quantitative Composition of Compounds111 Questions
Exam 8: Chemical Equations113 Questions
Exam 9: Calculations From Chemical Equations101 Questions
Exam 10: Modern Atomic Theory and the Periodic Table99 Questions
Exam 11: Chemical Bonds: the Formation of Compounds From Atoms102 Questions
Exam 12: The Gaseous State of Matter100 Questions
Exam 13: Liquids100 Questions
Exam 14: Solutions100 Questions
Exam 15: Acids, Bases, and Salts100 Questions
Exam 16: Chemical Equilibrium101 Questions
Exam 17: Oxidationreduction100 Questions
Exam 18: Nuclear Chemistry102 Questions
Exam 19: Introduction to Organic Chemistry Online Only99 Questions
Exam 20: Introduction to Biochemistry Online Only105 Questions
Select questions type
A base solution contains 0.400 moles of OH -1.The base solution is neutralized by 43.4 mL of sulfuric acid.What is the molarity of the sulfuric acid solution?
(Short Answer)
4.9/5  (36)
(36)
Which are the two Bronsted-Lowry acids in the following equation? H2S + H2O  HS -1 + H3O +1
HS -1 + H3O +1
(Multiple Choice)
4.8/5  (36)
(36)
What is the freezing point of a 3.0 m aqueous solution of NaCl? (The freezing point depression constant for water is 1.86 °C/m. )
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the pH of the following solutions:
A.0.034 M HCl
B.0.31 M HNO3
C.0.0034 M HI
(Short Answer)
4.8/5  (43)
(43)
What is the pH of a solution whose H3O +1 concentration is 1 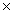 10 -9?
10 -9?
(Multiple Choice)
4.8/5  (35)
(35)
What is the concentration of calcium ion in a 2.0 M solution of calcium chloride?
(Multiple Choice)
4.9/5  (44)
(44)
For the following neutralization reaction:
hydrochloric acid plus barium hydroxide yields barium chloride plus water
A.Write the balanced formula equation.
B.Write the total ionic equation.
C.Write the net ionic equation.
(Short Answer)
4.9/5  (39)
(39)
The concentration of an aqueous solution of iron(II)chloride is 0.0550 M.What is the molarity of each ion in this solution?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)