Exam 4: Forces Between Particles
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
When magnesium forms an ion,it would be represented as _____ .
Free
(Multiple Choice)
4.7/5  (43)
(43)
Correct Answer:
D
K+ and Cl- have the same electronic configuration as argon.
Free
(True/False)
4.7/5  (40)
(40)
Correct Answer:
True
Which of the following electronic changes will cause a Group VA (15)element to achieve a noble gas configuration?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
Hydrogen bonds form between the molecules of the hypothetical compound ABCH and do not form between DEFH molecules.
(Multiple Choice)
4.7/5  (42)
(42)
A polyatomic ion is a species that has both ionic and covalent bonding.
(True/False)
4.9/5  (40)
(40)
During the formation of an ionic bond between Na and N,which of the following reactions occurs?
(Multiple Choice)
4.9/5  (36)
(36)
Compounds containing covalent bonds are more soluble in water than those with ionic bonds.
(True/False)
4.8/5  (37)
(37)
Which of the following Lewis structures depicts provides a reasonable representation of SO2?
(Multiple Choice)
4.8/5  (42)
(42)
Both bonding and non-bonding electron pairs can influence molecular shape.
(True/False)
4.9/5  (36)
(36)
The correct formula for the ionic compound containing Al3+ and PO 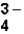 Would be _____.
Would be _____.
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following is a correct Lewis structure for a group IIIA(13)element M?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)