Exam 2: Atoms and Molecules
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
In naturally occurring samples,all elements exist as a mixture of isotopes.
Free
(True/False)
5.0/5  (38)
(38)
Correct Answer:
False
The atomic mass number is a whole number and indicates a specific isotope of an element.
Free
(True/False)
4.9/5  (35)
(35)
Correct Answer:
True
How many electrons are found around the species below? 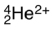
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
Which element is approximately 65 percent of sulfuric acid by weight?
(Multiple Choice)
4.9/5  (36)
(36)
Individuals,ages 19-70,should include at least 1000 mg of calcium in their daily diet.What is the minimum number of one-gram calcium supplement tablets you would need to take each day to meet this requirement if a tablet is 40% by mass calcium?
(Multiple Choice)
4.8/5  (32)
(32)
Using whole numbers,determine the molecular weight of calcium hydroxide,Ca(OH)2.
(Multiple Choice)
4.8/5  (39)
(39)
Write the formula for a compound consisting of 3 sodium atoms,1 phosphorus atom,and 4 oxygen atoms.
(Multiple Choice)
4.7/5  (35)
(35)
The most accurate way to determine atomic mass is with a mass spectrometer.
(True/False)
4.9/5  (33)
(33)
How many moles of hydrogen molecules (H2)would be required to produce two moles of hydrogen peroxide (H2O2)?
(Multiple Choice)
4.8/5  (33)
(33)
The average relative mass of an ozone molecule is 48.0 u.An ozone molecule contains only oxygen atoms.What does this molecular weight indicate about the formula of the ozone molecule?
(Multiple Choice)
4.7/5  (35)
(35)
Consider the representation shown below.It should be classified as 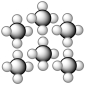
(Multiple Choice)
4.8/5  (42)
(42)
You discover a new element which consists of two isotopes.The first isotope,243X (mass = 242.45 u)comprises 40.000% of the total.The second isotope,248X (mass = 247.11 u)accounts for the rest.What would be the average atomic mass for your new element?
(Multiple Choice)
4.8/5  (41)
(41)
Approximately,how many atoms of beryllium would be required to equal the mass of 10 atoms of aluminum?
(Multiple Choice)
4.9/5  (44)
(44)
The formula for dinitrogen monoxide is N2O.If a sample of the oxide was found to contain 0.0800 g of oxygen,how many grams of nitrogen would it contain?
(Multiple Choice)
4.9/5  (38)
(38)
Oxalic acid is found in many plants,like spinach and black tea.What is the mass of the carbon found in one mole of oxalic acid,H2C2O4?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)