Exam 10: Radioactivity and Nuclear Processes
Exam 1: Matter, measurements, and Calculations92 Questions
Exam 2: Atoms and Molecules89 Questions
Exam 3: Electronic Structure and the Periodic Law86 Questions
Exam 4: Forces Between Particles89 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: The States of Matter89 Questions
Exam 7: Solutions and Colloids88 Questions
Exam 8: Reaction Rates and Equilibrium87 Questions
Exam 9: Acids,bases,and Salts86 Questions
Exam 10: Radioactivity and Nuclear Processes86 Questions
Exam 11: Organic Compounds: Alkanes87 Questions
Exam 12: Unsaturated Hydrocarbons88 Questions
Exam 13: Alcohols, phenols, and Ethers87 Questions
Exam 14: Aldehydes and Ketones86 Questions
Exam 15: Carboxylic Acids and Esters86 Questions
Exam 16: Amines and Amides85 Questions
Exam 17: Carbohydrates88 Questions
Exam 18: Lipids91 Questions
Exam 19: Proteins89 Questions
Exam 20: Enzymes88 Questions
Exam 21: Nucleic Acids and Protein Synthesis90 Questions
Exam 22: Nutrition and Energy for Life89 Questions
Exam 23: Carbohydrate Metabolism90 Questions
Exam 24: Lipid and Amino Acid Metabolism94 Questions
Exam 25: Body Fluids86 Questions
Select questions type
Tritium has a half-life of 12.5 years.If you had a sample of 8.00 grams of tritium today,how many grams of tritium would you have in 37.5 years?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
Gamma emissions can be stopped with the use of an electromagnetic field because of their dense charge and their mass.
Free
(True/False)
4.9/5  (39)
(39)
Correct Answer:
False
Which of the responses represent the missing particle in the following reaction? 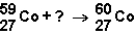
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
Sodium-24 has a half-life of 15.0 hours.Suppose you had a sample containing 0.010 moles of Na-24.How many hours would be required to reduce your sample to 6.25 *10-4 moles?
(Multiple Choice)
4.8/5  (38)
(38)
The radioisotope radon-222 is a gas that is a health hazard because it can make its way into the house from the soil in which it is produced,be inhaled,and cause lung cancer.
(True/False)
4.9/5  (44)
(44)
Sn-108 undergoes electron capture.The product of this reaction would be _____ .
(Multiple Choice)
4.7/5  (39)
(39)
Nuclear power plants have control rods that absorb neutrons to control the reaction.What is the composition of the control rods?
(Multiple Choice)
4.8/5  (32)
(32)
Which measure of radiation is used to account for health differences of various types of radiation?
(Multiple Choice)
4.9/5  (36)
(36)
An isotope of zinc containing 36 neutrons is bombarded with and captures a proton.What isotope is produced as a product of the reaction?
(Multiple Choice)
4.8/5  (29)
(29)
A radioactive isotope of a metal produced a reading of 188 Bq.Another reading of the activity was taken 4 hours later and the reading was 47.0 Bq.What is the half-life of this isotope?
(Multiple Choice)
4.9/5  (35)
(35)
A major problem with attempting to measure the effect of an exposure to emissions is that each different emission has a different effect on tissue due to the differences in penetration.
(True/False)
4.8/5  (43)
(43)
An individual would have to work from behind a special,thick protective wall when which kind of an emitter is involved?
(Multiple Choice)
4.7/5  (36)
(36)
Technetium-99m is used in medical diagnosis by injecting a solution and watch for the pattern of emissions.A 0.325 g sample was injected into a person,and the emission rate indicates that there are approximately 0.01016 grams of Tc-99 left.How much time has passed since the injection? Tc-99 has a half-life of 6 hours.
(Multiple Choice)
4.8/5  (32)
(32)
Extensive use of an MRI should be avoided as it is a source of significant nuclear radiation exposure.
(True/False)
4.8/5  (39)
(39)
If you are a medical professional and have a chance to be exposed to many sources of radiation,which of the following units to measure biological radiation will most likely be used to express your level of total exposure?
(Multiple Choice)
4.7/5  (40)
(40)
A patient that works at a nuclear power plant comes to you complaining of nausea and fatigue.You find out that he might have been exposed to a radiation source;what would you recommend be done?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following consists of a stream of charged particles?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)