Exam 4: Molecular Shape and Structure
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
How many lone pairs of electrons are there in the Lewis structure of azidocarbonamide,H2NC(O)NNC(O)NH2?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following has bond angles slightly less than 109 ?
(Multiple Choice)
4.9/5  (43)
(43)
The molecules OF2 and O3 both have bent shapes.What are the approximate bond angles in OF2 and O3,respectively?
(Multiple Choice)
4.8/5  (43)
(43)
Germanium is a semiconductor.Which of the following should be added in small amounts to produce a p-type semiconductor?
(Multiple Choice)
4.7/5  (42)
(42)
The structure of Tylenol is given below: 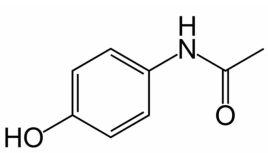 What hybrid orbitals are used on the N atom and the carbonyl carbon,respectively?
What hybrid orbitals are used on the N atom and the carbonyl carbon,respectively?
(Multiple Choice)
4.7/5  (38)
(38)
The fact that B2 has two unpaired electrons means the 2p molecular orbitals have higher energy than the 2p molecular orbitals.
(True/False)
4.8/5  (39)
(39)
Showing 21 - 40 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)