Exam 1: Matter, measurement, and Problem Solving
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Choose the pure substance from the list below.
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
B
Gas is sold for $1.399 per liter in Toronto,Canada.Your car needs 12.00 gallons.How much will your credit card be charged in Canadian dollars?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
The correct answer (reported to the proper number of significant figures)to the following is: 12.5 × 9.68 = ________
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following represents a chemical property of hydrogen gas?
(Multiple Choice)
4.7/5  (34)
(34)
How many liters of air are in a room that measures 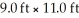 and has a 10.0 ft ceiling? 1 in.= 2.54 cm (exactly); 1 L = 103 cm3
and has a 10.0 ft ceiling? 1 in.= 2.54 cm (exactly); 1 L = 103 cm3
(Multiple Choice)
4.9/5  (46)
(46)
Read the length of the metal bar with the correct number of significant figures. 
(Multiple Choice)
4.8/5  (35)
(35)
What is the volume (in cm3)of a 43.6 g piece of metal with a density of 2.71 g/cm3?
(Multiple Choice)
4.9/5  (37)
(37)
If a solution has a temperature of 255 K,what is its temperature in degrees Celsius?
(Multiple Choice)
4.9/5  (31)
(31)
How many significant figures are there in the answer to the following problem? (9.992 × 3.200)+ 0.610 = ?
(Multiple Choice)
4.8/5  (35)
(35)
The outside air temperature is 40°F,what is the temperature in Kelvin?
(Multiple Choice)
4.8/5  (37)
(37)
An acetylene molecule contains 2 atoms of carbon.The number 2 represents how many significant figures?
(Multiple Choice)
4.8/5  (29)
(29)
Round the following number to four significant figures and express the result in standard exponential notation: 442,722
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)